| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Cytochrome P450 3A4 |
|---|
| Ligand | BDBM50430792 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_943410 (CHEMBL2341374) |
|---|
| IC50 | 7300±n/a nM |
|---|
| Citation |  Estrada, AA; Shore, DG; Blackwood, E; Chen, YH; Deshmukh, G; Ding, X; Dipasquale, AG; Epler, JA; Friedman, LS; Koehler, MF; Liu, L; Malek, S; Nonomiya, J; Ortwine, DF; Pei, Z; Sideris, S; St-Jean, F; Trinh, L; Truong, T; Lyssikatos, JP Pyrimidoaminotropanes as potent, selective, and efficacious small molecule kinase inhibitors of the mammalian target of rapamycin (mTOR). J Med Chem56:3090-101 (2013) [PubMed] Article Estrada, AA; Shore, DG; Blackwood, E; Chen, YH; Deshmukh, G; Ding, X; Dipasquale, AG; Epler, JA; Friedman, LS; Koehler, MF; Liu, L; Malek, S; Nonomiya, J; Ortwine, DF; Pei, Z; Sideris, S; St-Jean, F; Trinh, L; Truong, T; Lyssikatos, JP Pyrimidoaminotropanes as potent, selective, and efficacious small molecule kinase inhibitors of the mammalian target of rapamycin (mTOR). J Med Chem56:3090-101 (2013) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Cytochrome P450 3A4 |
|---|
| Name: | Cytochrome P450 3A4 |
|---|
| Synonyms: | Albendazole monooxygenase | Albendazole sulfoxidase | CP3A4_HUMAN | CYP3A3 | CYP3A4 | CYPIIIA3 | CYPIIIA4 | Cytochrome P450 3A3 | Cytochrome P450 3A4 (CYP3A4) | Cytochrome P450 HLp | Nifedipine oxidase | Quinine 3-monooxygenase | Taurochenodeoxycholate 6-alpha-hydroxylase |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 57349.57 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | n/a |
|---|
| Residue: | 503 |
|---|
| Sequence: | MALIPDLAMETWLLLAVSLVLLYLYGTHSHGLFKKLGIPGPTPLPFLGNILSYHKGFCMF
DMECHKKYGKVWGFYDGQQPVLAITDPDMIKTVLVKECYSVFTNRRPFGPVGFMKSAISI
AEDEEWKRLRSLLSPTFTSGKLKEMVPIIAQYGDVLVRNLRREAETGKPVTLKDVFGAYS
MDVITSTSFGVNIDSLNNPQDPFVENTKKLLRFDFLDPFFLSITVFPFLIPILEVLNICV
FPREVTNFLRKSVKRMKESRLEDTQKHRVDFLQLMIDSQNSKETESHKALSDLELVAQSI
IFIFAGYETTSSVLSFIMYELATHPDVQQKLQEEIDAVLPNKAPPTYDTVLQMEYLDMVV
NETLRLFPIAMRLERVCKKDVEINGMFIPKGVVVMIPSYALHRDPKYWTEPEKFLPERFS
KKNKDNIDPYIYTPFGSGPRNCIGMRFALMNMKLALIRVLQNFSFKPCKETQIPLKLSLG
GLLQPEKPVVLKVESRDGTVSGA
|
|
|
|---|
| BDBM50430792 |
|---|
| n/a |
|---|
| Name | BDBM50430792 |
|---|
| Synonyms: | CHEMBL2334759 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C25H32N6O3 |
|---|
| Mol. Mass. | 464.56 |
|---|
| SMILES | CCNC(=O)Nc1ccc(cc1)-c1nc2C[C@@H]3CC[C@@H](N3C(C)=O)c2c(n1)N1CCOC[C@@H]1C |r,THB:21:20:14.24.15:18.17| |
|---|
| Structure | 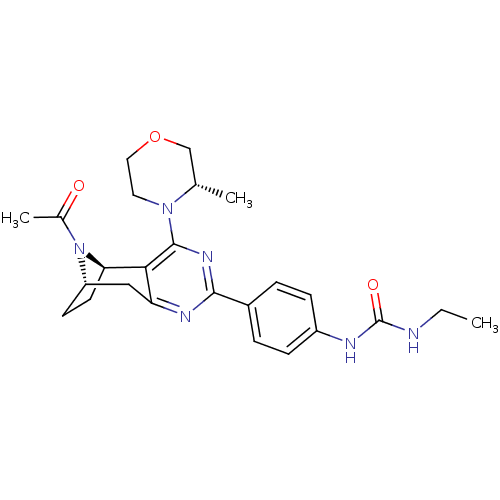
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Estrada, AA; Shore, DG; Blackwood, E; Chen, YH; Deshmukh, G; Ding, X; Dipasquale, AG; Epler, JA; Friedman, LS; Koehler, MF; Liu, L; Malek, S; Nonomiya, J; Ortwine, DF; Pei, Z; Sideris, S; St-Jean, F; Trinh, L; Truong, T; Lyssikatos, JP Pyrimidoaminotropanes as potent, selective, and efficacious small molecule kinase inhibitors of the mammalian target of rapamycin (mTOR). J Med Chem56:3090-101 (2013) [PubMed] Article
Estrada, AA; Shore, DG; Blackwood, E; Chen, YH; Deshmukh, G; Ding, X; Dipasquale, AG; Epler, JA; Friedman, LS; Koehler, MF; Liu, L; Malek, S; Nonomiya, J; Ortwine, DF; Pei, Z; Sideris, S; St-Jean, F; Trinh, L; Truong, T; Lyssikatos, JP Pyrimidoaminotropanes as potent, selective, and efficacious small molecule kinase inhibitors of the mammalian target of rapamycin (mTOR). J Med Chem56:3090-101 (2013) [PubMed] Article