

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM50086681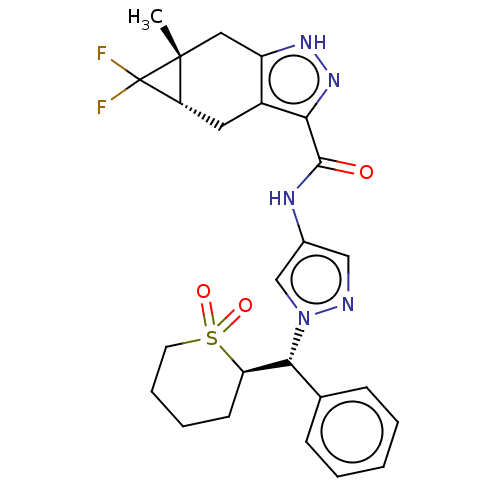 (CHEMBL3426309) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc. Curated by ChEMBL | Assay Description Inhibition of GST-tagged recombinant full length human ITK using AcEFPIYDFLPAKKK-NH2 as substrate after 35 mins by Morrison plot analysis | J Med Chem 58: 3806-16 (2015) Article DOI: 10.1021/jm501998m BindingDB Entry DOI: 10.7270/Q28K7BTH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM50015266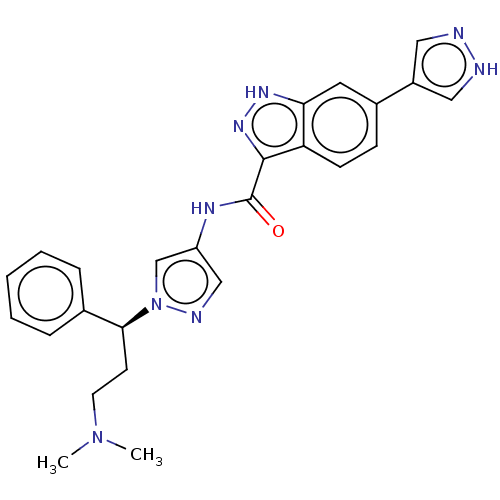 (CHEMBL3263053) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc. Curated by ChEMBL | Assay Description Inhibition of GST-tagged full length ITK (unknown origin) using BLK peptide as substrate after 35 mins | J Med Chem 57: 5714-27 (2014) Article DOI: 10.1021/jm500550e BindingDB Entry DOI: 10.7270/Q2ZK5J7T | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM50015266 (CHEMBL3263053) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc. Curated by ChEMBL | Assay Description Inhibition of full-length ITK (unknown origin) using biotin-EQEDEPEGIYGVLF-NH2 as substrate by plate reader analysis | Bioorg Med Chem Lett 24: 2448-52 (2014) Article DOI: 10.1016/j.bmcl.2014.04.023 BindingDB Entry DOI: 10.7270/Q2W097G8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM50037076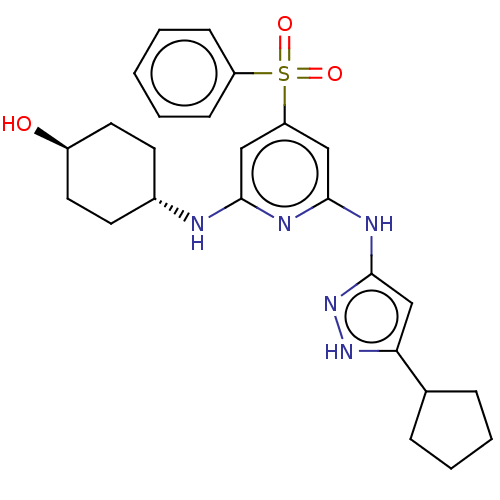 (CHEMBL3355737) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Evotec UK Ltd Curated by ChEMBL | Assay Description Inhibition of GST-tagged full-length ITK (unknown origin) using Ac-EFPIYDFLPAKKK-NH2 as substrate after 35 mins by LC/MS analysis | Bioorg Med Chem Lett 24: 5818-23 (2014) Article DOI: 10.1016/j.bmcl.2014.10.020 BindingDB Entry DOI: 10.7270/Q2NK3GNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM50022940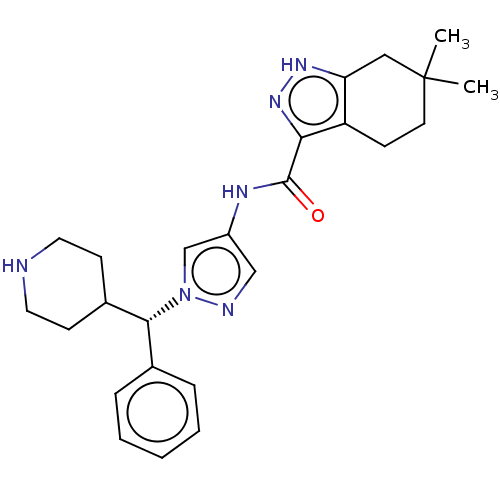 (CHEMBL3298373) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc. Curated by ChEMBL | Assay Description Inhibition of GST-tagged full length ITK (unknown origin) using BLK peptide as substrate after 35 mins | J Med Chem 57: 5714-27 (2014) Article DOI: 10.1021/jm500550e BindingDB Entry DOI: 10.7270/Q2ZK5J7T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM50086604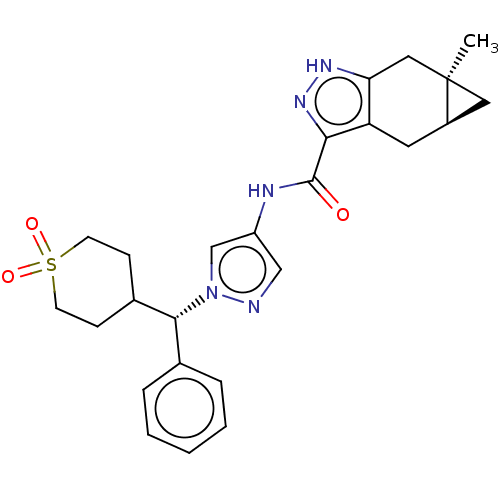 (CHEMBL3426308) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc. Curated by ChEMBL | Assay Description Inhibition of GST-tagged recombinant full length human ITK using AcEFPIYDFLPAKKK-NH2 as substrate after 35 mins by Morrison plot analysis | J Med Chem 58: 3806-16 (2015) Article DOI: 10.1021/jm501998m BindingDB Entry DOI: 10.7270/Q28K7BTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM50015268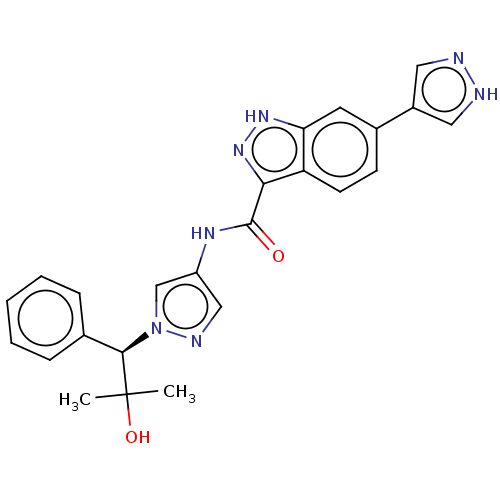 (CHEMBL3263055) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc. Curated by ChEMBL | Assay Description Inhibition of full-length ITK (unknown origin) using biotin-EQEDEPEGIYGVLF-NH2 as substrate by plate reader analysis | Bioorg Med Chem Lett 24: 2448-52 (2014) Article DOI: 10.1016/j.bmcl.2014.04.023 BindingDB Entry DOI: 10.7270/Q2W097G8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM50037066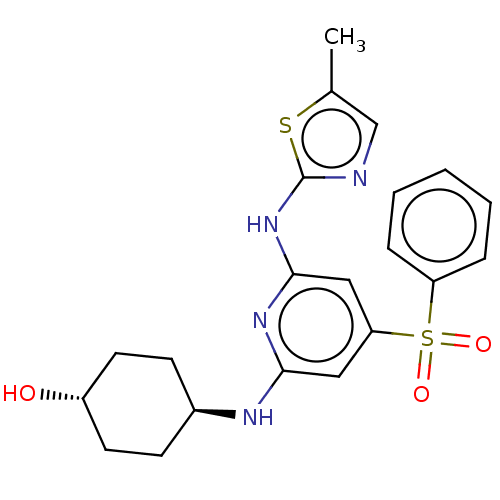 (CHEMBL3355728) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Evotec UK Ltd Curated by ChEMBL | Assay Description Inhibition of GST-tagged full-length ITK (unknown origin) using Ac-EFPIYDFLPAKKK-NH2 as substrate after 35 mins by LC/MS analysis | Bioorg Med Chem Lett 24: 5818-23 (2014) Article DOI: 10.1016/j.bmcl.2014.10.020 BindingDB Entry DOI: 10.7270/Q2NK3GNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM50086676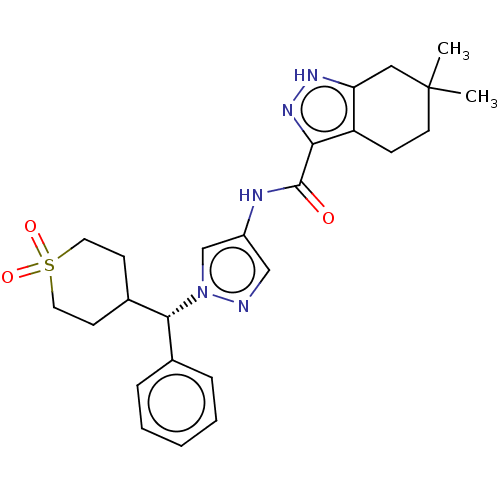 (CHEMBL3426304) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc. Curated by ChEMBL | Assay Description Inhibition of GST-tagged recombinant full length human ITK using AcEFPIYDFLPAKKK-NH2 as substrate after 35 mins by Morrison plot analysis | J Med Chem 58: 3806-16 (2015) Article DOI: 10.1021/jm501998m BindingDB Entry DOI: 10.7270/Q28K7BTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM50086671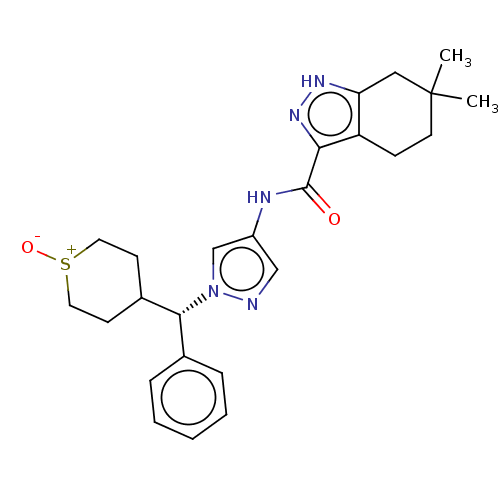 (CHEMBL3426305) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc. Curated by ChEMBL | Assay Description Inhibition of GST-tagged recombinant full length human ITK using AcEFPIYDFLPAKKK-NH2 as substrate after 35 mins by Morrison plot analysis | J Med Chem 58: 3806-16 (2015) Article DOI: 10.1021/jm501998m BindingDB Entry DOI: 10.7270/Q28K7BTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM50086671 (CHEMBL3426305) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc. Curated by ChEMBL | Assay Description Inhibition of GST-tagged recombinant full length human ITK using AcEFPIYDFLPAKKK-NH2 as substrate after 35 mins by Morrison plot analysis | J Med Chem 58: 3806-16 (2015) Article DOI: 10.1021/jm501998m BindingDB Entry DOI: 10.7270/Q28K7BTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM50086659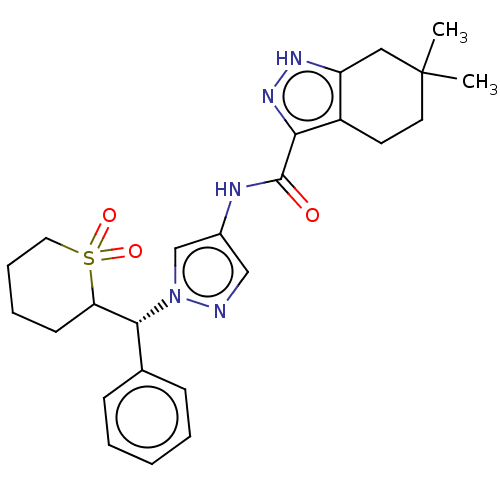 (CHEMBL3426303) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc. Curated by ChEMBL | Assay Description Inhibition of GST-tagged recombinant full length human ITK using AcEFPIYDFLPAKKK-NH2 as substrate after 35 mins by Morrison plot analysis | J Med Chem 58: 3806-16 (2015) Article DOI: 10.1021/jm501998m BindingDB Entry DOI: 10.7270/Q28K7BTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM50086659 (CHEMBL3426303) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc. Curated by ChEMBL | Assay Description Inhibition of GST-tagged recombinant full length human ITK using AcEFPIYDFLPAKKK-NH2 as substrate after 35 mins by Morrison plot analysis | J Med Chem 58: 3806-16 (2015) Article DOI: 10.1021/jm501998m BindingDB Entry DOI: 10.7270/Q28K7BTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM50086605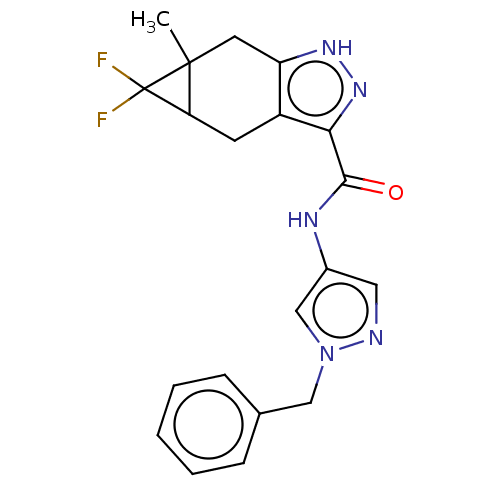 (CHEMBL3426307) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc. Curated by ChEMBL | Assay Description Inhibition of GST-tagged recombinant full length human ITK using AcEFPIYDFLPAKKK-NH2 as substrate after 35 mins by Morrison plot analysis | J Med Chem 58: 3806-16 (2015) Article DOI: 10.1021/jm501998m BindingDB Entry DOI: 10.7270/Q28K7BTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM50343775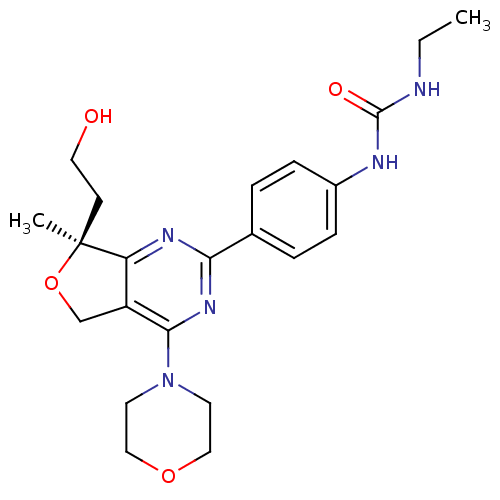 ((S)-1-ethyl-3-(4-(7-(2-hydroxyethyl)-7-methyl-4-mo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of human recombinant mTOR expressed in insect cells using 4E-BP1 substrate after 30 mins by fluorescence resonance energy transfer assay | J Med Chem 54: 3426-35 (2011) Article DOI: 10.1021/jm200215y BindingDB Entry DOI: 10.7270/Q29C6XRH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM50037077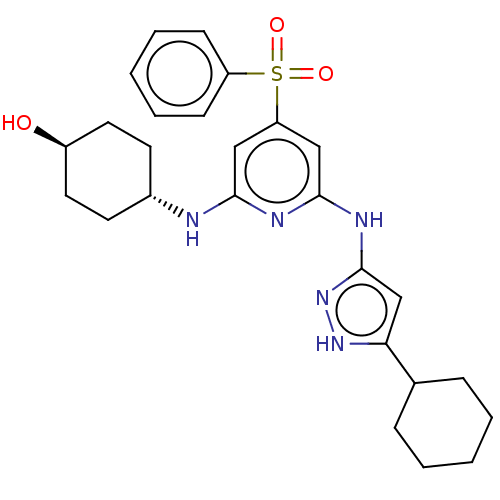 (CHEMBL3355738) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Evotec UK Ltd Curated by ChEMBL | Assay Description Inhibition of GST-tagged full-length ITK (unknown origin) using Ac-EFPIYDFLPAKKK-NH2 as substrate after 35 mins by LC/MS analysis | Bioorg Med Chem Lett 24: 5818-23 (2014) Article DOI: 10.1016/j.bmcl.2014.10.020 BindingDB Entry DOI: 10.7270/Q2NK3GNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM50428131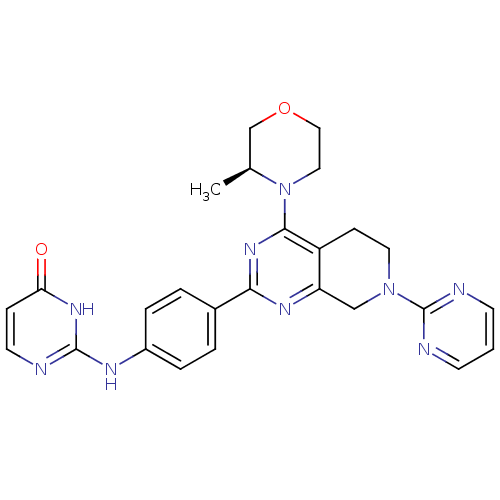 (CHEMBL2331687) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of recombinant mTOR (1360 to 2549)+GBL (unknown origin) using GFP-4E-BP1 as substrate after 30 mins by FRET assay | ACS Med Chem Lett 4: 103-7 (2013) Article DOI: 10.1021/ml3003132 BindingDB Entry DOI: 10.7270/Q28C9XK3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11108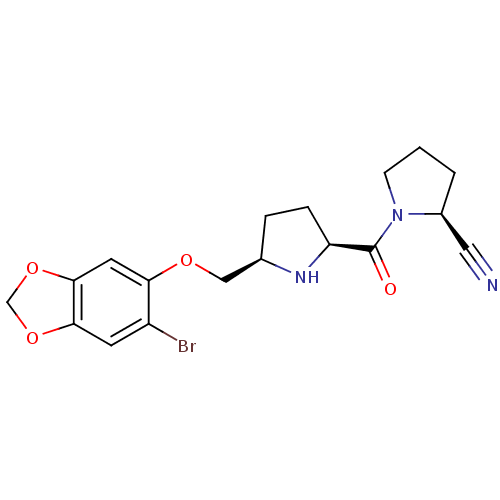 ((2S)-1-{[(2S,5R)-5-{[(6-bromo-2H-1,3-benzodioxol-5...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The DPP4 activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 355 nm and mea... | J Med Chem 49: 3520-35 (2006) Article DOI: 10.1021/jm051283e BindingDB Entry DOI: 10.7270/Q2WS8RG1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11103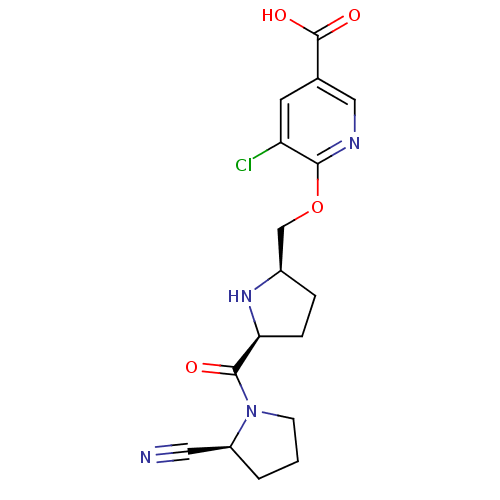 (2-cyanopyrrolidine 21aj | 5-chloro-6-{[(2R,5S)-5-{...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The DPP4 activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 355 nm and mea... | J Med Chem 49: 3520-35 (2006) Article DOI: 10.1021/jm051283e BindingDB Entry DOI: 10.7270/Q2WS8RG1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM50443182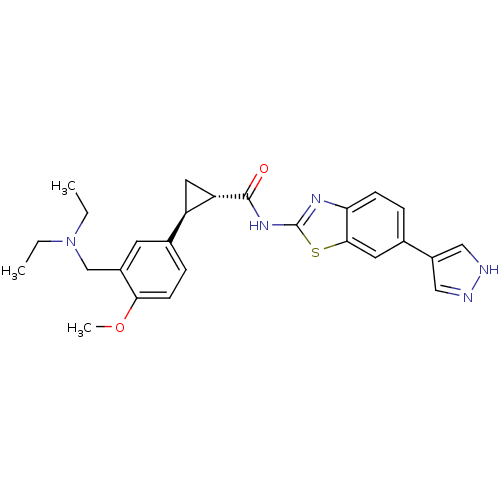 (CHEMBL3086538) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Evotec (UK) Ltd Curated by ChEMBL | Assay Description Inhibition of full length GST-tagged ITK (unknown origin) using Ac-EFPIYDFLPAKKK-NH2 as substrate after 35 mins by LC/MS analysis | Bioorg Med Chem Lett 23: 6331-5 (2013) Article DOI: 10.1016/j.bmcl.2013.09.069 BindingDB Entry DOI: 10.7270/Q2GX4D16 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM50086680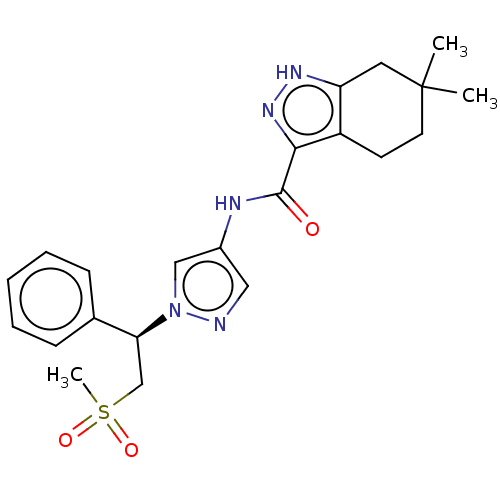 (CHEMBL3426306) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc. Curated by ChEMBL | Assay Description Inhibition of GST-tagged recombinant full length human ITK using AcEFPIYDFLPAKKK-NH2 as substrate after 35 mins by Morrison plot analysis | J Med Chem 58: 3806-16 (2015) Article DOI: 10.1021/jm501998m BindingDB Entry DOI: 10.7270/Q28K7BTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM50015263 (CHEMBL3263050) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc. Curated by ChEMBL | Assay Description Inhibition of full-length ITK (unknown origin) using biotin-EQEDEPEGIYGVLF-NH2 as substrate by plate reader analysis | Bioorg Med Chem Lett 24: 2448-52 (2014) Article DOI: 10.1016/j.bmcl.2014.04.023 BindingDB Entry DOI: 10.7270/Q2W097G8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11087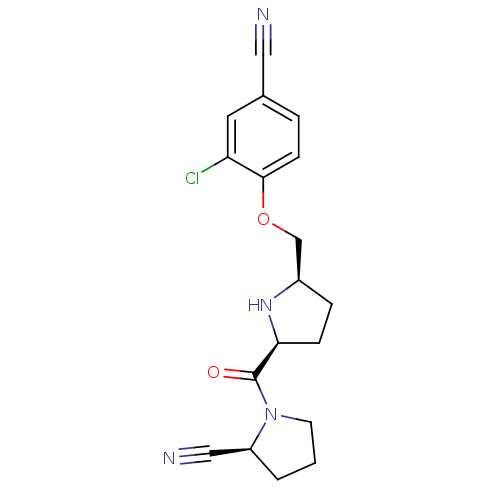 ((2S)-1-{[(2S,5R)-5-(2-chloro-4-cyanophenoxymethyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The DPP4 activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 355 nm and mea... | J Med Chem 49: 3520-35 (2006) Article DOI: 10.1021/jm051283e BindingDB Entry DOI: 10.7270/Q2WS8RG1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM50443181 (CHEMBL3086535) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Evotec (UK) Ltd Curated by ChEMBL | Assay Description Inhibition of full length GST-tagged ITK (unknown origin) using Ac-EFPIYDFLPAKKK-NH2 as substrate after 35 mins by LC/MS analysis | Bioorg Med Chem Lett 23: 6331-5 (2013) Article DOI: 10.1016/j.bmcl.2013.09.069 BindingDB Entry DOI: 10.7270/Q2GX4D16 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11110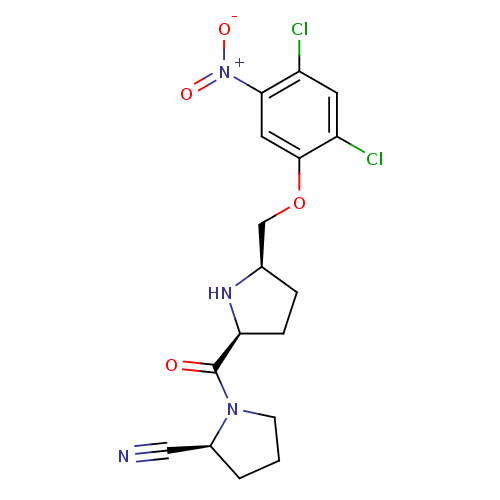 ((2S)-1-{[(2S,5R)-5-(2,4-dichloro-5-nitrophenoxymet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The DPP4 activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 355 nm and mea... | J Med Chem 49: 3520-35 (2006) Article DOI: 10.1021/jm051283e BindingDB Entry DOI: 10.7270/Q2WS8RG1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11101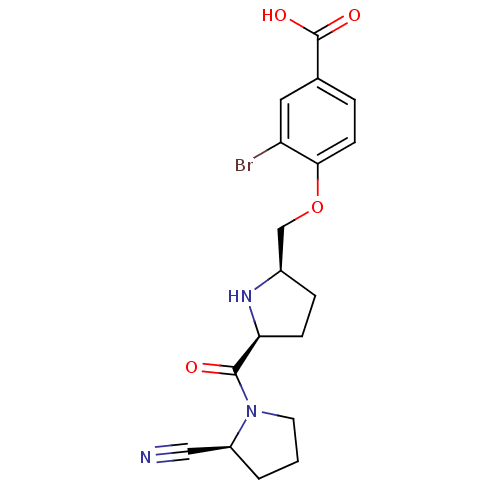 (2-cyanopyrrolidine 21ah | 3-bromo-4-{[(2R,5S)-5-{[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The DPP4 activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 355 nm and mea... | J Med Chem 49: 3520-35 (2006) Article DOI: 10.1021/jm051283e BindingDB Entry DOI: 10.7270/Q2WS8RG1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11112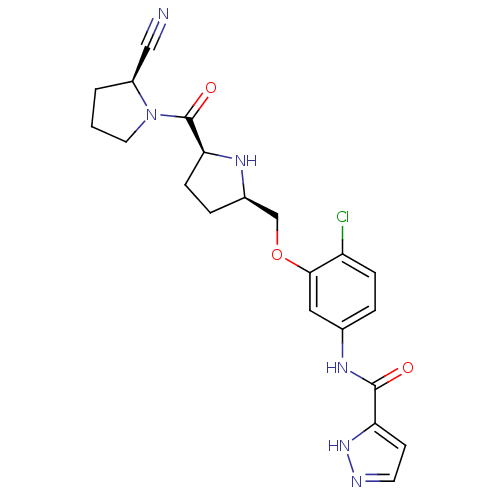 (2-cyanopyrrolidine 21as | N-(4-chloro-3-{[(2R,5S)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The DPP4 activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 355 nm and mea... | J Med Chem 49: 3520-35 (2006) Article DOI: 10.1021/jm051283e BindingDB Entry DOI: 10.7270/Q2WS8RG1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM50343776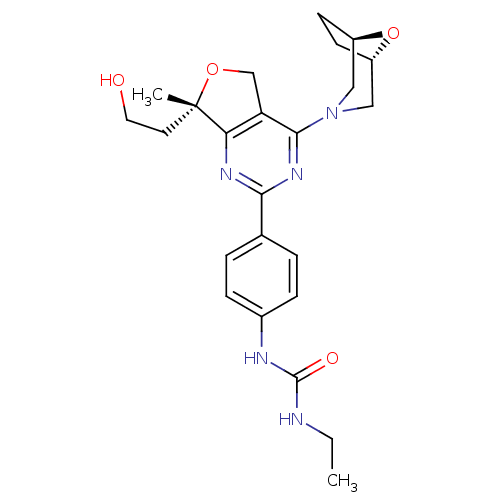 (1-(4-((S)-4-((1R,5S)-8-oxa-3-azabicyclo[3.2.1]octa...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of human recombinant mTOR expressed in insect cells using 4E-BP1 substrate after 30 mins by fluorescence resonance energy transfer assay | J Med Chem 54: 3426-35 (2011) Article DOI: 10.1021/jm200215y BindingDB Entry DOI: 10.7270/Q29C6XRH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11107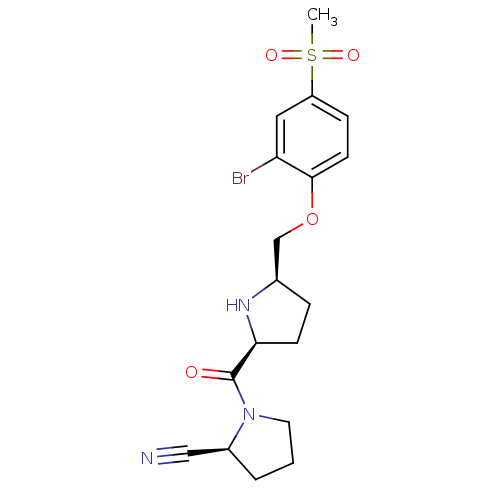 ((2S)-1-{[(2S,5R)-5-(2-bromo-4-methanesulfonylpheno...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The DPP4 activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 355 nm and mea... | J Med Chem 49: 3520-35 (2006) Article DOI: 10.1021/jm051283e BindingDB Entry DOI: 10.7270/Q2WS8RG1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11104 (2-cyanopyrrolidine 21ak | 6-chloro-5-{[(2R,5S)-5-{...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The DPP4 activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 355 nm and mea... | J Med Chem 49: 3520-35 (2006) Article DOI: 10.1021/jm051283e BindingDB Entry DOI: 10.7270/Q2WS8RG1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM50022934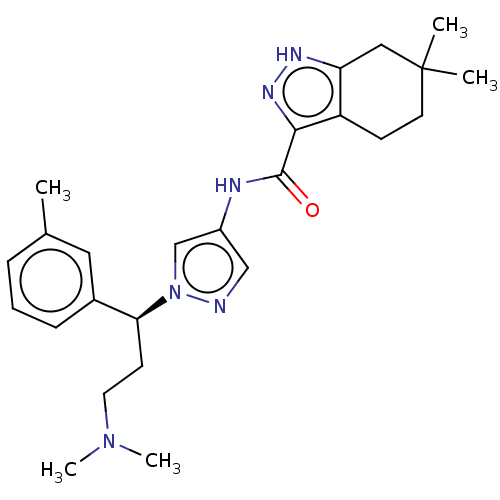 (CHEMBL3298375) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc. Curated by ChEMBL | Assay Description Inhibition of GST-tagged full length ITK (unknown origin) using BLK peptide as substrate after 35 mins | J Med Chem 57: 5714-27 (2014) Article DOI: 10.1021/jm500550e BindingDB Entry DOI: 10.7270/Q2ZK5J7T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM50443180 (CHEMBL3086536) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Evotec (UK) Ltd Curated by ChEMBL | Assay Description Inhibition of full length GST-tagged ITK (unknown origin) using Ac-EFPIYDFLPAKKK-NH2 as substrate after 35 mins by LC/MS analysis | Bioorg Med Chem Lett 23: 6331-5 (2013) Article DOI: 10.1016/j.bmcl.2013.09.069 BindingDB Entry DOI: 10.7270/Q2GX4D16 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM50343774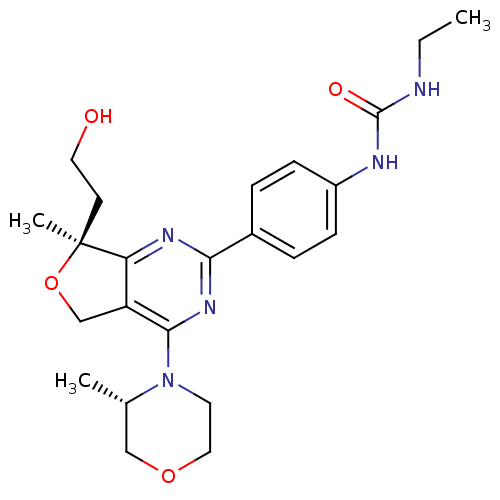 (1-Ethyl-3-(4-((S)-7-(2-hydroxyethyl)-7-methyl-4-((...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of human recombinant mTOR expressed in insect cells using 4E-BP1 substrate after 30 mins by fluorescence resonance energy transfer assay | J Med Chem 54: 3426-35 (2011) Article DOI: 10.1021/jm200215y BindingDB Entry DOI: 10.7270/Q29C6XRH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM50022932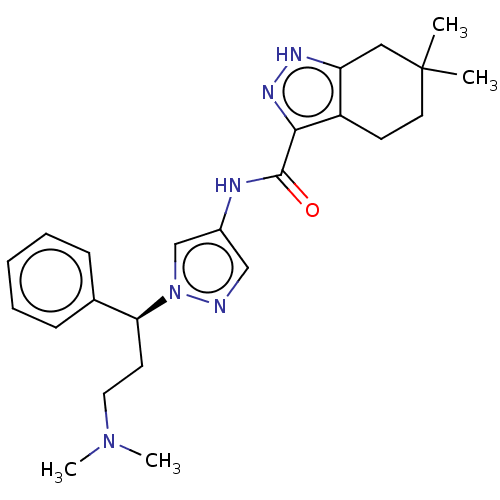 (CHEMBL3298371) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc. Curated by ChEMBL | Assay Description Inhibition of GST-tagged full length ITK (unknown origin) using BLK peptide as substrate after 35 mins | J Med Chem 57: 5714-27 (2014) Article DOI: 10.1021/jm500550e BindingDB Entry DOI: 10.7270/Q2ZK5J7T | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11090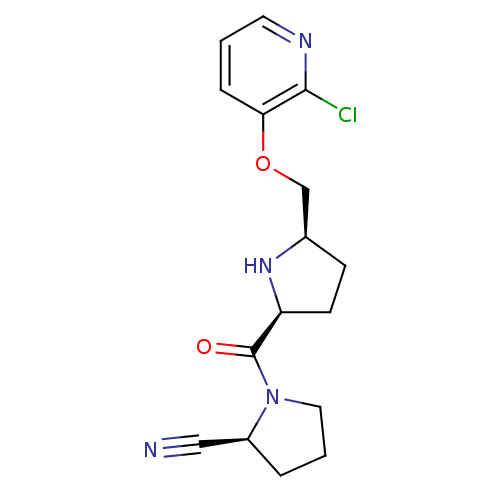 ((2S)-1-{[(2S,5R)-5-{[(2-chloropyridin-3-yl)oxy]met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | 0.720 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The DPP4 activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 355 nm and mea... | J Med Chem 49: 3520-35 (2006) Article DOI: 10.1021/jm051283e BindingDB Entry DOI: 10.7270/Q2WS8RG1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11111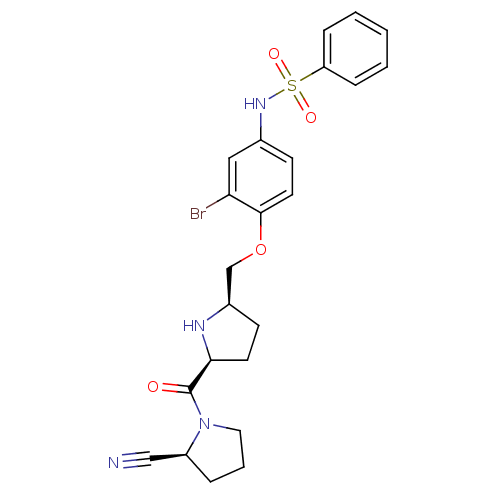 (2-cyanopyrrolidine 21ar | N-(3-bromo-4-{[(2R,5S)-5...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.730 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The DPP4 activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 355 nm and mea... | J Med Chem 49: 3520-35 (2006) Article DOI: 10.1021/jm051283e BindingDB Entry DOI: 10.7270/Q2WS8RG1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM50015271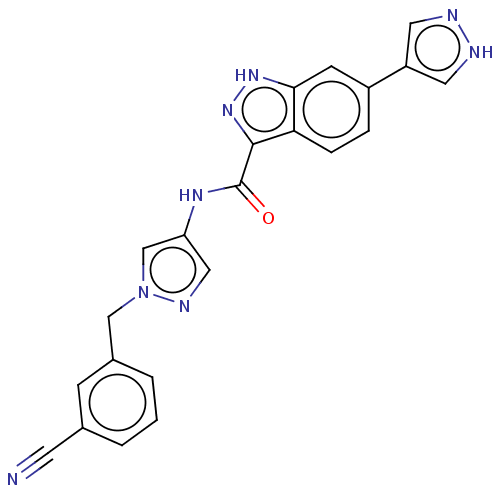 (CHEMBL3263036) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc. Curated by ChEMBL | Assay Description Inhibition of full-length ITK (unknown origin) using biotin-EQEDEPEGIYGVLF-NH2 as substrate by plate reader analysis | Bioorg Med Chem Lett 24: 2448-52 (2014) Article DOI: 10.1016/j.bmcl.2014.04.023 BindingDB Entry DOI: 10.7270/Q2W097G8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM50015267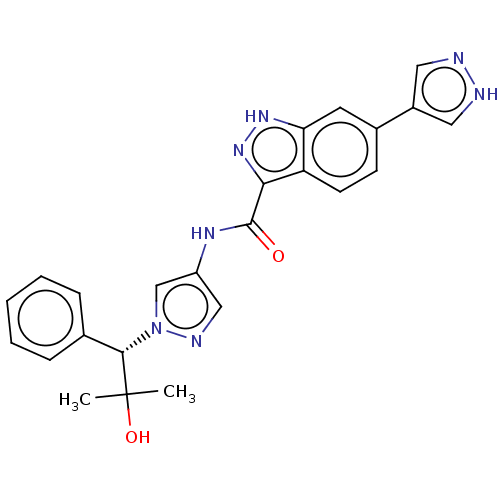 (CHEMBL3263054) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc. Curated by ChEMBL | Assay Description Inhibition of full-length ITK (unknown origin) using biotin-EQEDEPEGIYGVLF-NH2 as substrate by plate reader analysis | Bioorg Med Chem Lett 24: 2448-52 (2014) Article DOI: 10.1016/j.bmcl.2014.04.023 BindingDB Entry DOI: 10.7270/Q2W097G8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM50022927 (CHEMBL3298370) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc. Curated by ChEMBL | Assay Description Inhibition of GST-tagged full length ITK (unknown origin) using BLK peptide as substrate after 35 mins | J Med Chem 57: 5714-27 (2014) Article DOI: 10.1021/jm500550e BindingDB Entry DOI: 10.7270/Q2ZK5J7T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM50343769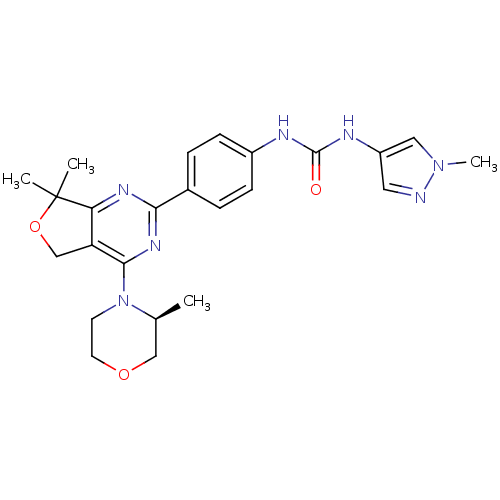 ((S)-1-(4-(7,7-Dimethyl-4-(3-methylmorpholino)-5,7-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of human recombinant mTOR expressed in insect cells using 4E-BP1 substrate after 30 mins by fluorescence resonance energy transfer assay | J Med Chem 54: 3426-35 (2011) Article DOI: 10.1021/jm200215y BindingDB Entry DOI: 10.7270/Q29C6XRH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11100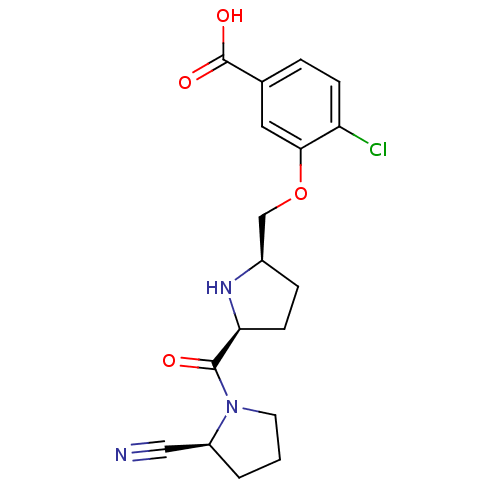 (2-cyanopyrrolidine 21ag | 4-chloro-3-{[(2R,5S)-5-{...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.820 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The DPP4 activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 355 nm and mea... | J Med Chem 49: 3520-35 (2006) Article DOI: 10.1021/jm051283e BindingDB Entry DOI: 10.7270/Q2WS8RG1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11085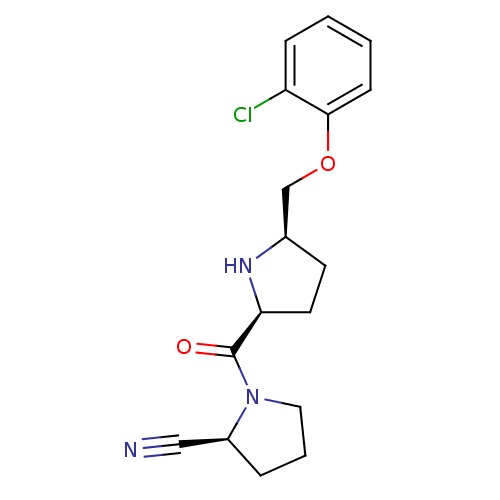 ((2S)-1-{[(2S,5R)-5-(2-chlorophenoxymethyl)pyrrolid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The DPP4 activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 355 nm and mea... | J Med Chem 49: 3520-35 (2006) Article DOI: 10.1021/jm051283e BindingDB Entry DOI: 10.7270/Q2WS8RG1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM50086669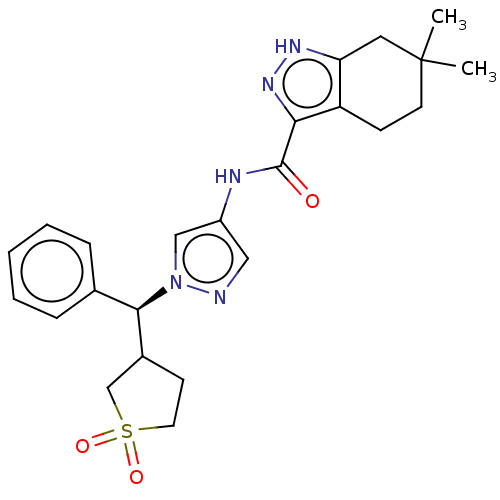 (CHEMBL3426301) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc. Curated by ChEMBL | Assay Description Inhibition of GST-tagged recombinant full length human ITK using AcEFPIYDFLPAKKK-NH2 as substrate after 35 mins by Morrison plot analysis | J Med Chem 58: 3806-16 (2015) Article DOI: 10.1021/jm501998m BindingDB Entry DOI: 10.7270/Q28K7BTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM50086669 (CHEMBL3426301) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc. Curated by ChEMBL | Assay Description Inhibition of GST-tagged recombinant full length human ITK using AcEFPIYDFLPAKKK-NH2 as substrate after 35 mins by Morrison plot analysis | J Med Chem 58: 3806-16 (2015) Article DOI: 10.1021/jm501998m BindingDB Entry DOI: 10.7270/Q28K7BTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11098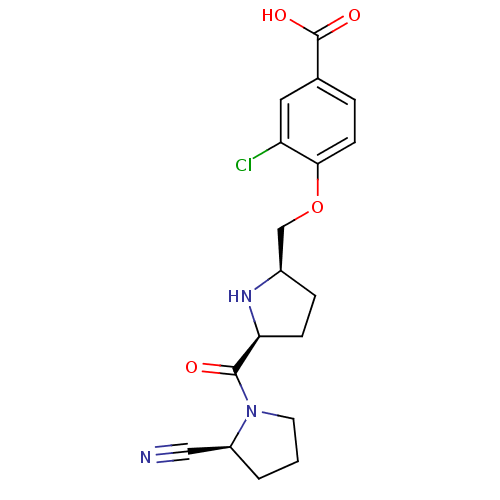 (2-cyanopyrrolidine 21ae | 3-chloro-4-{[(2R,5S)-5-{...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.930 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The DPP4 activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 355 nm and mea... | J Med Chem 49: 3520-35 (2006) Article DOI: 10.1021/jm051283e BindingDB Entry DOI: 10.7270/Q2WS8RG1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM50015265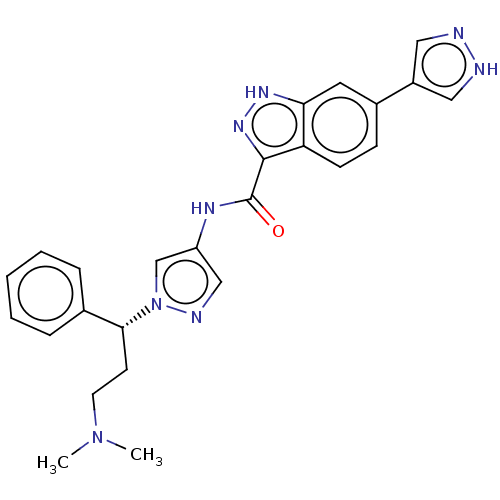 (CHEMBL3263052) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.930 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc. Curated by ChEMBL | Assay Description Inhibition of full-length ITK (unknown origin) using biotin-EQEDEPEGIYGVLF-NH2 as substrate by plate reader analysis | Bioorg Med Chem Lett 24: 2448-52 (2014) Article DOI: 10.1016/j.bmcl.2014.04.023 BindingDB Entry DOI: 10.7270/Q2W097G8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM50430787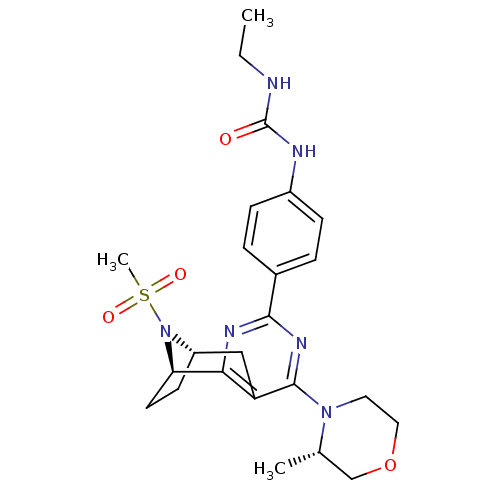 (CHEMBL2334764) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.990 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of human recombinant mTOR expressed in insect cells assessed as inhibition of phosphorylation of (GFP)-4-EBP1 protein after 30 mins by flu... | J Med Chem 56: 3090-101 (2013) Article DOI: 10.1021/jm400194n BindingDB Entry DOI: 10.7270/Q2QJ7JNF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11644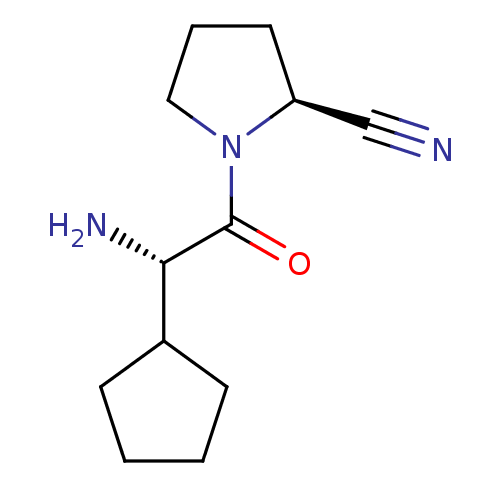 ((2S)-1-[(2S)-2-amino-2-cyclopentylacetyl]pyrrolidi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1 | -50.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description The DPP4 activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 355 nm and mea... | J Med Chem 49: 6416-20 (2006) Article DOI: 10.1021/jm060777o BindingDB Entry DOI: 10.7270/Q2M043M1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM50439517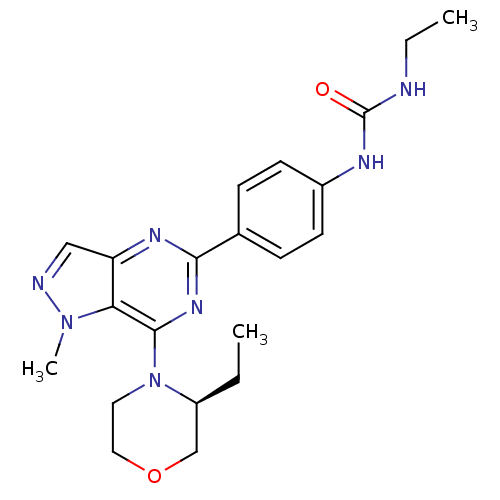 (CHEMBL2418349) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of human recombinant mTOR (1360-2549) expressed in insect cells assessed as phosphorylation of recombinant (GFP)-4-EBP1 after 30 mins by L... | Bioorg Med Chem Lett 23: 5097-104 (2013) Article DOI: 10.1016/j.bmcl.2013.07.027 BindingDB Entry DOI: 10.7270/Q2QN687D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM50015262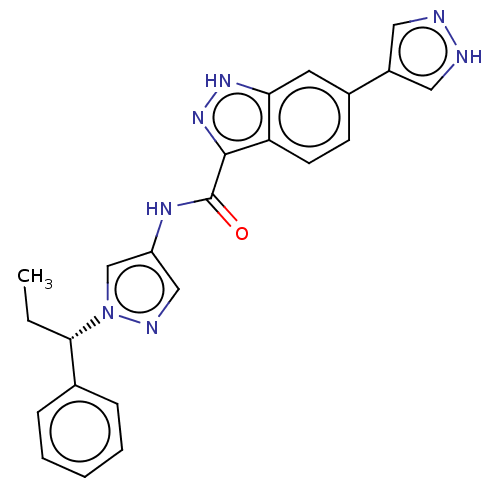 (CHEMBL3263049) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc. Curated by ChEMBL | Assay Description Inhibition of full-length ITK (unknown origin) using biotin-EQEDEPEGIYGVLF-NH2 as substrate by plate reader analysis | Bioorg Med Chem Lett 24: 2448-52 (2014) Article DOI: 10.1016/j.bmcl.2014.04.023 BindingDB Entry DOI: 10.7270/Q2W097G8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 3437 total ) | Next | Last >> |