

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Reaction Details | |||
|---|---|---|---|
 | Report a problem with these data | ||
| Target | Prostaglandin E synthase | ||
| Ligand | BDBM50425318 | ||
| Substrate/Competitor | n/a | ||
| Meas. Tech. | ChEMBL_964545 (CHEMBL2393812) | ||
| IC50 | 36±n/a nM | ||
| Citation |  Arhancet, GB; Walker, DP; Metz, S; Fobian, YM; Heasley, SE; Carter, JS; Springer, JR; Jones, DE; Hayes, MJ; Shaffer, AF; Jerome, GM; Baratta, MT; Zweifel, B; Moore, WM; Masferrer, JL; Vazquez, ML Discovery and SAR of PF-4693627, a potent, selective and orally bioavailable mPGES-1 inhibitor for the potential treatment of inflammation. Bioorg Med Chem Lett23:1114-9 (2013) [PubMed] Article Arhancet, GB; Walker, DP; Metz, S; Fobian, YM; Heasley, SE; Carter, JS; Springer, JR; Jones, DE; Hayes, MJ; Shaffer, AF; Jerome, GM; Baratta, MT; Zweifel, B; Moore, WM; Masferrer, JL; Vazquez, ML Discovery and SAR of PF-4693627, a potent, selective and orally bioavailable mPGES-1 inhibitor for the potential treatment of inflammation. Bioorg Med Chem Lett23:1114-9 (2013) [PubMed] Article | ||
| More Info.: | Get all data from this article, Assay Method | ||
| Prostaglandin E synthase | |||
| Name: | Prostaglandin E synthase | ||
| Synonyms: | MGST1L1 | MPGES1 | PGES | PIG12 | PTGES | PTGES_HUMAN | Prostaglandin E synthase (PGES-1) | Prostaglandin E synthase 1 (mPGES-1) | Prostaglandin E synthase-1 (PGES-1) | Prostaglandin E synthase/G/H synthase 2 | Prostaglandin E2 synthase-1 ( mPGES-1) | ||
| Type: | Protein | ||
| Mol. Mass.: | 17112.22 | ||
| Organism: | Homo sapiens (Human) | ||
| Description: | n/a | ||
| Residue: | 152 | ||
| Sequence: |
| ||
| BDBM50425318 | |||
| n/a | |||
| Name | BDBM50425318 | ||
| Synonyms: | CHEMBL2315861 | ||
| Type | Small organic molecule | ||
| Emp. Form. | C20H26ClN3O3 | ||
| Mol. Mass. | 391.892 | ||
| SMILES | OC[C@H]1CC[C@H](CC1)NC(=O)C1CCN(CC1)c1nc2cc(Cl)ccc2o1 |r,wD:5.8,2.1,(21.18,-14.42,;20.41,-13.09,;18.87,-13.08,;18.1,-11.75,;16.56,-11.75,;15.8,-13.08,;16.57,-14.42,;18.1,-14.42,;14.27,-13.08,;13.49,-11.75,;14.26,-10.42,;11.95,-11.75,;11.18,-13.09,;9.65,-13.09,;8.88,-11.77,;9.63,-10.43,;11.18,-10.42,;7.34,-11.77,;6.44,-13.03,;4.97,-12.56,;3.63,-13.34,;2.3,-12.57,;.96,-13.34,;2.3,-11.02,;3.63,-10.25,;4.96,-11.02,;6.43,-10.53,)| | ||
| Structure | 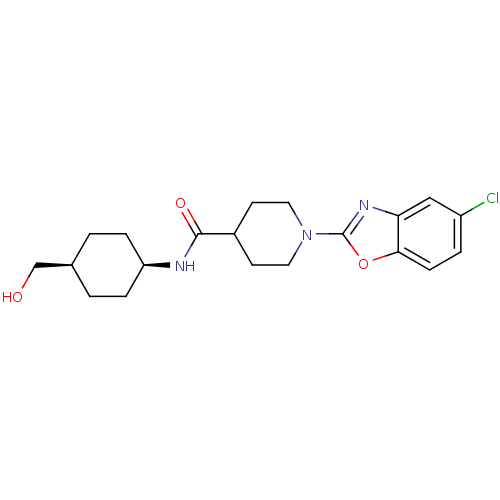
| ||