 Found 251 hits with Last Name = 'metz' and Initial = 's'
Found 251 hits with Last Name = 'metz' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM7167

(3-(4-sulfamoylphenyl)-3,4,10,11-tetraazatricyclo[7...)Show SMILES NC(=O)c1nn(-c2ccc(cc2)S(N)(=O)=O)c2c1ccc1[nH]ncc21 Show InChI InChI=1S/C15H12N6O3S/c16-15(22)13-10-5-6-12-11(7-18-19-12)14(10)21(20-13)8-1-3-9(4-2-8)25(17,23)24/h1-7H,(H2,16,22)(H,18,19)(H2,17,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Nerviano Medical Sciences
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence ATP/[gamma-33P] ATP. After in... |
Bioorg Med Chem Lett 15: 1315-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.01.023
BindingDB Entry DOI: 10.7270/Q2N58JKH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM7177

(3-(4-methanesulfonylphenyl)-3,4,10,11-tetraazatric...)Show SMILES CS(=O)(=O)c1ccc(cc1)-n1nc(C(N)=O)c2ccc3[nH]ncc3c12 Show InChI InChI=1S/C16H13N5O3S/c1-25(23,24)10-4-2-9(3-5-10)21-15-11(14(20-21)16(17)22)6-7-13-12(15)8-18-19-13/h2-8H,1H3,(H2,17,22)(H,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Nerviano Medical Sciences
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence ATP/[gamma-33P] ATP. After in... |
Bioorg Med Chem Lett 15: 1315-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.01.023
BindingDB Entry DOI: 10.7270/Q2N58JKH |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50435048
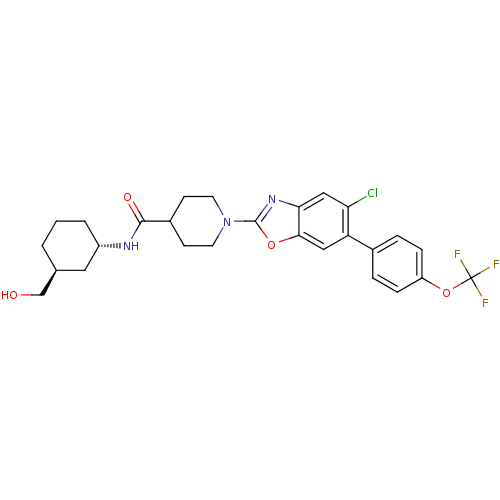
(CHEMBL2391142)Show SMILES OC[C@H]1CCC[C@@H](C1)NC(=O)C1CCN(CC1)c1nc2cc(Cl)c(cc2o1)-c1ccc(OC(F)(F)F)cc1 |r| Show InChI InChI=1S/C27H29ClF3N3O4/c28-22-14-23-24(13-21(22)17-4-6-20(7-5-17)38-27(29,30)31)37-26(33-23)34-10-8-18(9-11-34)25(36)32-19-3-1-2-16(12-19)15-35/h4-7,13-14,16,18-19,35H,1-3,8-12,15H2,(H,32,36)/t16-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mPGES-1 (unknown origin) using PGH2 as substrate assessed as PGE2 synthesis by ELISA |
Bioorg Med Chem Lett 23: 1114-9 (2013)
Article DOI: 10.1016/j.bmcl.2012.11.109
BindingDB Entry DOI: 10.7270/Q2FQ9Z0W |
More data for this
Ligand-Target Pair | |
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM7214

(3-(2,2,2-trifluoroethyl)-3,4,10,11-tetraazatricycl...)Show InChI InChI=1S/C11H8F3N5O/c12-11(13,14)4-19-9-5(8(18-19)10(15)20)1-2-7-6(9)3-16-17-7/h1-3H,4H2,(H2,15,20)(H,16,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence ATP/[gamma-33P] ATP. After in... |
Bioorg Med Chem Lett 15: 1315-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.01.023
BindingDB Entry DOI: 10.7270/Q2N58JKH |
More data for this
Ligand-Target Pair | |
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM7169
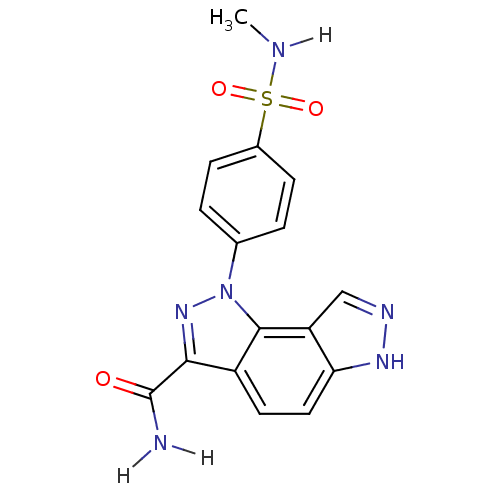
(3-[4-(methylsulfamoyl)phenyl]-3,4,10,11-tetraazatr...)Show SMILES CNS(=O)(=O)c1ccc(cc1)-n1nc(C(N)=O)c2ccc3[nH]ncc3c12 Show InChI InChI=1S/C16H14N6O3S/c1-18-26(24,25)10-4-2-9(3-5-10)22-15-11(14(21-22)16(17)23)6-7-13-12(15)8-19-20-13/h2-8,18H,1H3,(H2,17,23)(H,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Nerviano Medical Sciences
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence ATP/[gamma-33P] ATP. After in... |
Bioorg Med Chem Lett 15: 1315-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.01.023
BindingDB Entry DOI: 10.7270/Q2N58JKH |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM7173

(3-[4-(butylsulfamoyl)phenyl]-3,4,10,11-tetraazatri...)Show SMILES CCCCNS(=O)(=O)c1ccc(cc1)-n1nc(C(N)=O)c2ccc3[nH]ncc3c12 Show InChI InChI=1S/C19H20N6O3S/c1-2-3-10-22-29(27,28)13-6-4-12(5-7-13)25-18-14(17(24-25)19(20)26)8-9-16-15(18)11-21-23-16/h4-9,11,22H,2-3,10H2,1H3,(H2,20,26)(H,21,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Nerviano Medical Sciences
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence ATP/[gamma-33P] ATP. After in... |
Bioorg Med Chem Lett 15: 1315-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.01.023
BindingDB Entry DOI: 10.7270/Q2N58JKH |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50426967
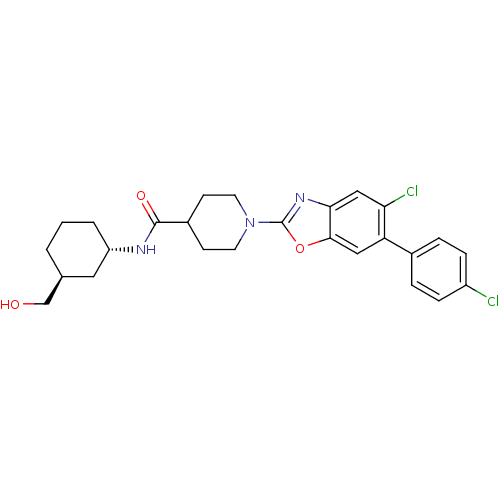
(CHEMBL2325079 | PF-4693627)Show SMILES OC[C@H]1CCC[C@@H](C1)NC(=O)C1CCN(CC1)c1nc2cc(Cl)c(cc2o1)-c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C26H29Cl2N3O3/c27-19-6-4-17(5-7-19)21-13-24-23(14-22(21)28)30-26(34-24)31-10-8-18(9-11-31)25(33)29-20-3-1-2-16(12-20)15-32/h4-7,13-14,16,18,20,32H,1-3,8-12,15H2,(H,29,33)/t16-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human mPGES1 by ELISA |
Bioorg Med Chem Lett 23: 1120-6 (2013)
Article DOI: 10.1016/j.bmcl.2012.11.107
BindingDB Entry DOI: 10.7270/Q26W9CDS |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50435045
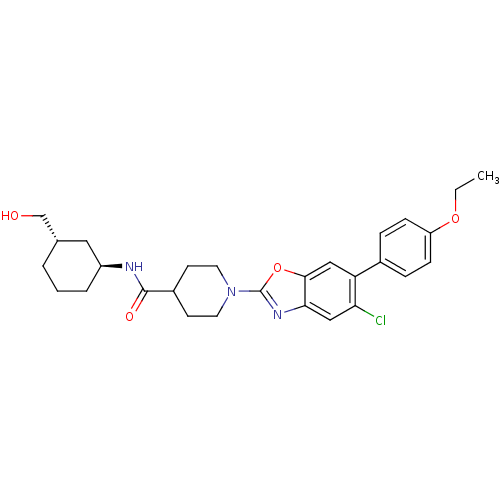
(CHEMBL2391141)Show SMILES CCOc1ccc(cc1)-c1cc2oc(nc2cc1Cl)N1CCC(CC1)C(=O)N[C@H]1CCC[C@H](CO)C1 |r| Show InChI InChI=1S/C28H34ClN3O4/c1-2-35-22-8-6-19(7-9-22)23-15-26-25(16-24(23)29)31-28(36-26)32-12-10-20(11-13-32)27(34)30-21-5-3-4-18(14-21)17-33/h6-9,15-16,18,20-21,33H,2-5,10-14,17H2,1H3,(H,30,34)/t18-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mPGES-1 (unknown origin) using PGH2 as substrate assessed as PGE2 synthesis by ELISA |
Bioorg Med Chem Lett 23: 1114-9 (2013)
Article DOI: 10.1016/j.bmcl.2012.11.109
BindingDB Entry DOI: 10.7270/Q2FQ9Z0W |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50435047
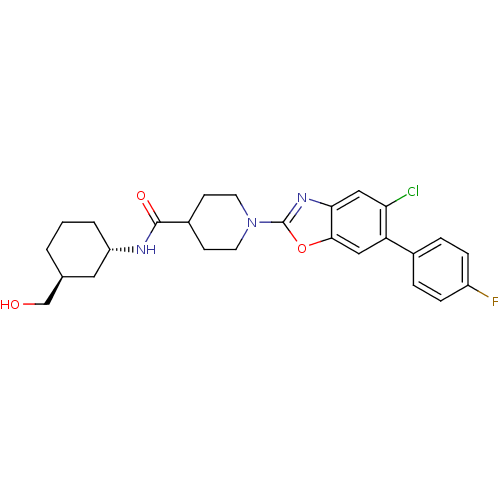
(CHEMBL2391299)Show SMILES OC[C@H]1CCC[C@@H](C1)NC(=O)C1CCN(CC1)c1nc2cc(Cl)c(cc2o1)-c1ccc(F)cc1 |r| Show InChI InChI=1S/C26H29ClFN3O3/c27-22-14-23-24(13-21(22)17-4-6-19(28)7-5-17)34-26(30-23)31-10-8-18(9-11-31)25(33)29-20-3-1-2-16(12-20)15-32/h4-7,13-14,16,18,20,32H,1-3,8-12,15H2,(H,29,33)/t16-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mPGES-1 (unknown origin) using PGH2 as substrate assessed as PGE2 synthesis by ELISA |
Bioorg Med Chem Lett 23: 1114-9 (2013)
Article DOI: 10.1016/j.bmcl.2012.11.109
BindingDB Entry DOI: 10.7270/Q2FQ9Z0W |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50426967

(CHEMBL2325079 | PF-4693627)Show SMILES OC[C@H]1CCC[C@@H](C1)NC(=O)C1CCN(CC1)c1nc2cc(Cl)c(cc2o1)-c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C26H29Cl2N3O3/c27-19-6-4-17(5-7-19)21-13-24-23(14-22(21)28)30-26(34-24)31-10-8-18(9-11-31)25(33)29-20-3-1-2-16(12-20)15-32/h4-7,13-14,16,18,20,32H,1-3,8-12,15H2,(H,29,33)/t16-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mPGES-1 (unknown origin) using PGH2 as substrate assessed as PGE2 synthesis by ELISA |
Bioorg Med Chem Lett 23: 1114-9 (2013)
Article DOI: 10.1016/j.bmcl.2012.11.109
BindingDB Entry DOI: 10.7270/Q2FQ9Z0W |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50435046
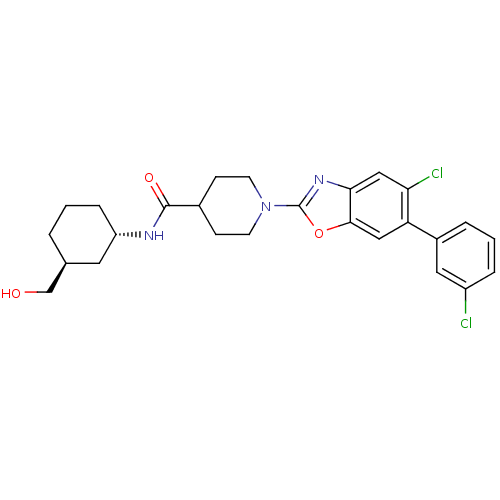
(CHEMBL2391140)Show SMILES OC[C@H]1CCC[C@@H](C1)NC(=O)C1CCN(CC1)c1nc2cc(Cl)c(cc2o1)-c1cccc(Cl)c1 |r| Show InChI InChI=1S/C26H29Cl2N3O3/c27-19-5-2-4-18(12-19)21-13-24-23(14-22(21)28)30-26(34-24)31-9-7-17(8-10-31)25(33)29-20-6-1-3-16(11-20)15-32/h2,4-5,12-14,16-17,20,32H,1,3,6-11,15H2,(H,29,33)/t16-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mPGES-1 (unknown origin) using PGH2 as substrate assessed as PGE2 synthesis by ELISA |
Bioorg Med Chem Lett 23: 1114-9 (2013)
Article DOI: 10.1016/j.bmcl.2012.11.109
BindingDB Entry DOI: 10.7270/Q2FQ9Z0W |
More data for this
Ligand-Target Pair | |
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM7166
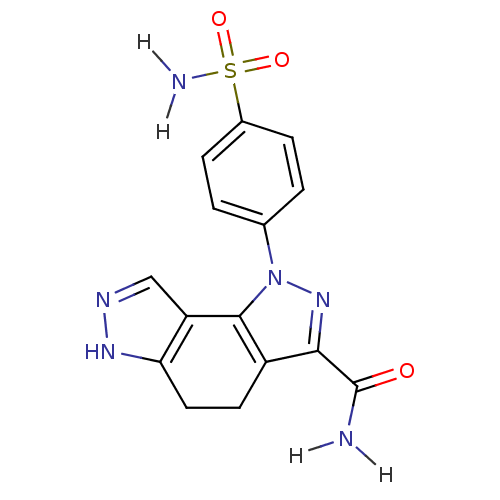
(3-(4-sulfamoylphenyl)-3,4,10,11-tetraazatricyclo[7...)Show SMILES NC(=O)c1nn(c-2c1CCc1[nH]ncc-21)-c1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C15H14N6O3S/c16-15(22)13-10-5-6-12-11(7-18-19-12)14(10)21(20-13)8-1-3-9(4-2-8)25(17,23)24/h1-4,7H,5-6H2,(H2,16,22)(H,18,19)(H2,17,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Nerviano Medical Sciences
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence ATP/[gamma-33P] ATP. After in... |
Bioorg Med Chem Lett 15: 1315-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.01.023
BindingDB Entry DOI: 10.7270/Q2N58JKH |
More data for this
Ligand-Target Pair | |
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM7195

(3-(4-cyanophenyl)-3,4,10,11-tetraazatricyclo[7.3.0...)Show SMILES NC(=O)c1nn(-c2ccc(cc2)C#N)c2c1ccc1[nH]ncc21 Show InChI InChI=1S/C16H10N6O/c17-7-9-1-3-10(4-2-9)22-15-11(14(21-22)16(18)23)5-6-13-12(15)8-19-20-13/h1-6,8H,(H2,18,23)(H,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence ATP/[gamma-33P] ATP. After in... |
Bioorg Med Chem Lett 15: 1315-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.01.023
BindingDB Entry DOI: 10.7270/Q2N58JKH |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50435044

(CHEMBL2391295)Show SMILES CC(C)c1cc2oc(nc2cc1Cl)N1CCC(CC1)C(=O)N[C@H]1CCC[C@H](CO)C1 |r| Show InChI InChI=1S/C23H32ClN3O3/c1-14(2)18-11-21-20(12-19(18)24)26-23(30-21)27-8-6-16(7-9-27)22(29)25-17-5-3-4-15(10-17)13-28/h11-12,14-17,28H,3-10,13H2,1-2H3,(H,25,29)/t15-,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mPGES-1 (unknown origin) using PGH2 as substrate assessed as PGE2 synthesis by ELISA |
Bioorg Med Chem Lett 23: 1114-9 (2013)
Article DOI: 10.1016/j.bmcl.2012.11.109
BindingDB Entry DOI: 10.7270/Q2FQ9Z0W |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50426967

(CHEMBL2325079 | PF-4693627)Show SMILES OC[C@H]1CCC[C@@H](C1)NC(=O)C1CCN(CC1)c1nc2cc(Cl)c(cc2o1)-c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C26H29Cl2N3O3/c27-19-6-4-17(5-7-19)21-13-24-23(14-22(21)28)30-26(34-24)31-10-8-18(9-11-31)25(33)29-20-3-1-2-16(12-20)15-32/h4-7,13-14,16,18,20,32H,1-3,8-12,15H2,(H,29,33)/t16-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mPGES-1 in human fetal fibroblast cells assessed as PGF2alpha level after 50 mins by ELISA |
Bioorg Med Chem Lett 23: 1114-9 (2013)
Article DOI: 10.1016/j.bmcl.2012.11.109
BindingDB Entry DOI: 10.7270/Q2FQ9Z0W |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50425313
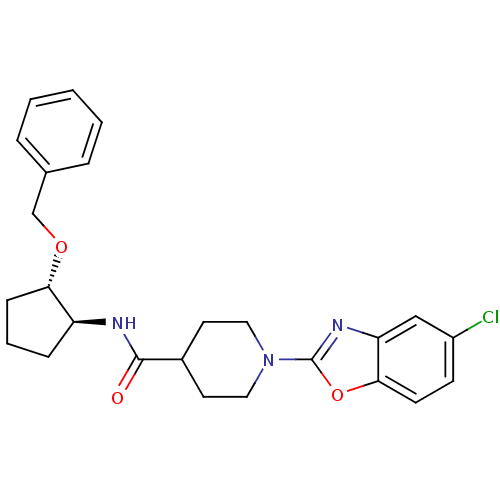
(CHEMBL2315853)Show SMILES Clc1ccc2oc(nc2c1)N1CCC(CC1)C(=O)N[C@H]1CCC[C@@H]1OCc1ccccc1 |r| Show InChI InChI=1S/C25H28ClN3O3/c26-19-9-10-23-21(15-19)28-25(32-23)29-13-11-18(12-14-29)24(30)27-20-7-4-8-22(20)31-16-17-5-2-1-3-6-17/h1-3,5-6,9-10,15,18,20,22H,4,7-8,11-14,16H2,(H,27,30)/t20-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mPGES-1-mediated PGE2 production in LPS-stimulated healthy human whole blood after 20 to 24 hrs by ELISA |
Bioorg Med Chem Lett 23: 1114-9 (2013)
Article DOI: 10.1016/j.bmcl.2012.11.109
BindingDB Entry DOI: 10.7270/Q2FQ9Z0W |
More data for this
Ligand-Target Pair | |
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM7187
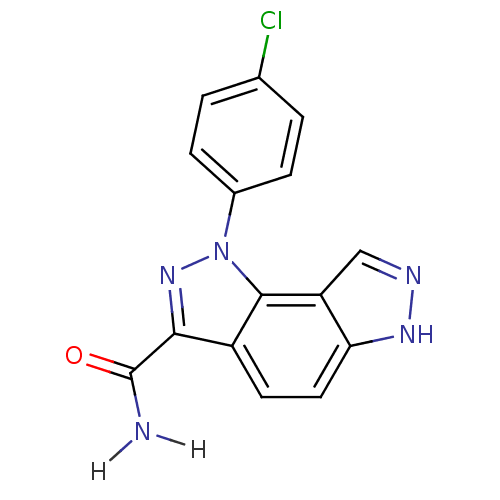
(3-(4-chlorophenyl)-3,4,10,11-tetraazatricyclo[7.3....)Show InChI InChI=1S/C15H10ClN5O/c16-8-1-3-9(4-2-8)21-14-10(13(20-21)15(17)22)5-6-12-11(14)7-18-19-12/h1-7H,(H2,17,22)(H,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence ATP/[gamma-33P] ATP. After in... |
Bioorg Med Chem Lett 15: 1315-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.01.023
BindingDB Entry DOI: 10.7270/Q2N58JKH |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM7206

(3-(pyridin-2-yl)-3,4,10,11-tetraazatricyclo[7.3.0....)Show InChI InChI=1S/C14H10N6O/c15-14(21)12-8-4-5-10-9(7-17-18-10)13(8)20(19-12)11-3-1-2-6-16-11/h1-7H,(H2,15,21)(H,17,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence ATP/[gamma-33P] ATP. After in... |
Bioorg Med Chem Lett 15: 1315-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.01.023
BindingDB Entry DOI: 10.7270/Q2N58JKH |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50426956
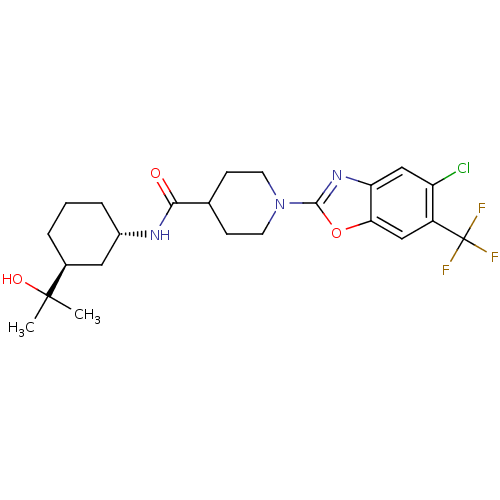
(CHEMBL2325076)Show SMILES CC(C)(O)[C@H]1CCC[C@@H](C1)NC(=O)C1CCN(CC1)c1nc2cc(Cl)c(cc2o1)C(F)(F)F |r| Show InChI InChI=1S/C23H29ClF3N3O3/c1-22(2,32)14-4-3-5-15(10-14)28-20(31)13-6-8-30(9-7-13)21-29-18-12-17(24)16(23(25,26)27)11-19(18)33-21/h11-15,32H,3-10H2,1-2H3,(H,28,31)/t14-,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human mPGES1 by ELISA |
Bioorg Med Chem Lett 23: 1120-6 (2013)
Article DOI: 10.1016/j.bmcl.2012.11.107
BindingDB Entry DOI: 10.7270/Q26W9CDS |
More data for this
Ligand-Target Pair | |
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM7185

(3-(4-methoxyphenyl)-3,4,10,11-tetraazatricyclo[7.3...)Show InChI InChI=1S/C16H13N5O2/c1-23-10-4-2-9(3-5-10)21-15-11(14(20-21)16(17)22)6-7-13-12(15)8-18-19-13/h2-8H,1H3,(H2,17,22)(H,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence ATP/[gamma-33P] ATP. After in... |
Bioorg Med Chem Lett 15: 1315-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.01.023
BindingDB Entry DOI: 10.7270/Q2N58JKH |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM7200

(3-(3-methylphenyl)-3,4,10,11-tetraazatricyclo[7.3....)Show InChI InChI=1S/C16H13N5O/c1-9-3-2-4-10(7-9)21-15-11(14(20-21)16(17)22)5-6-13-12(15)8-18-19-13/h2-8H,1H3,(H2,17,22)(H,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence ATP/[gamma-33P] ATP. After in... |
Bioorg Med Chem Lett 15: 1315-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.01.023
BindingDB Entry DOI: 10.7270/Q2N58JKH |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM7171
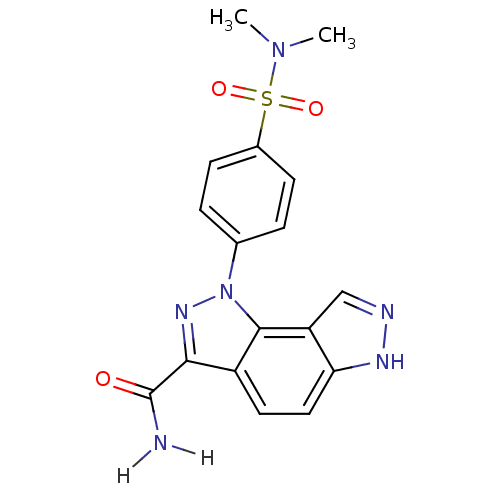
(3-[4-(dimethylsulfamoyl)phenyl]-3,4,10,11-tetraaza...)Show SMILES CN(C)S(=O)(=O)c1ccc(cc1)-n1nc(C(N)=O)c2ccc3[nH]ncc3c12 Show InChI InChI=1S/C17H16N6O3S/c1-22(2)27(25,26)11-5-3-10(4-6-11)23-16-12(15(21-23)17(18)24)7-8-14-13(16)9-19-20-14/h3-9H,1-2H3,(H2,18,24)(H,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Nerviano Medical Sciences
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence ATP/[gamma-33P] ATP. After in... |
Bioorg Med Chem Lett 15: 1315-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.01.023
BindingDB Entry DOI: 10.7270/Q2N58JKH |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM7191

(3-[4-(trifluoromethyl)phenyl]-3,4,10,11-tetraazatr...)Show SMILES NC(=O)c1nn(-c2ccc(cc2)C(F)(F)F)c2c1ccc1[nH]ncc21 Show InChI InChI=1S/C16H10F3N5O/c17-16(18,19)8-1-3-9(4-2-8)24-14-10(13(23-24)15(20)25)5-6-12-11(14)7-21-22-12/h1-7H,(H2,20,25)(H,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence ATP/[gamma-33P] ATP. After in... |
Bioorg Med Chem Lett 15: 1315-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.01.023
BindingDB Entry DOI: 10.7270/Q2N58JKH |
More data for this
Ligand-Target Pair | |
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM7181

(3-phenyl-3,4,10,11-tetraazatricyclo[7.3.0.0^{2,6}]...)Show InChI InChI=1S/C15H11N5O/c16-15(21)13-10-6-7-12-11(8-17-18-12)14(10)20(19-13)9-4-2-1-3-5-9/h1-8H,(H2,16,21)(H,17,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Nerviano Medical Sciences
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence ATP/[gamma-33P] ATP. After in... |
Bioorg Med Chem Lett 15: 1315-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.01.023
BindingDB Entry DOI: 10.7270/Q2N58JKH |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50426958
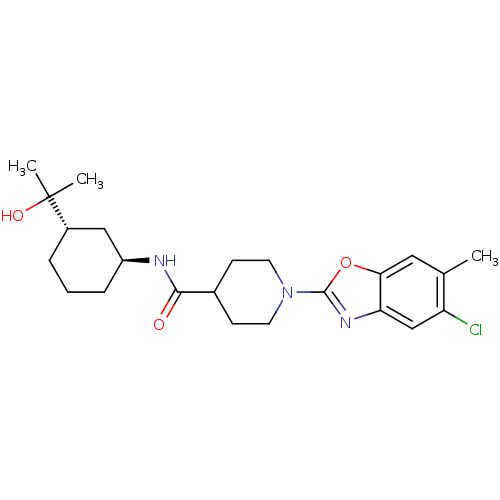
(CHEMBL2325074)Show SMILES Cc1cc2oc(nc2cc1Cl)N1CCC(CC1)C(=O)N[C@H]1CCC[C@@H](C1)C(C)(C)O |r| Show InChI InChI=1S/C23H32ClN3O3/c1-14-11-20-19(13-18(14)24)26-22(30-20)27-9-7-15(8-10-27)21(28)25-17-6-4-5-16(12-17)23(2,3)29/h11,13,15-17,29H,4-10,12H2,1-3H3,(H,25,28)/t16-,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human mPGES1 by ELISA |
Bioorg Med Chem Lett 23: 1120-6 (2013)
Article DOI: 10.1016/j.bmcl.2012.11.107
BindingDB Entry DOI: 10.7270/Q26W9CDS |
More data for this
Ligand-Target Pair | |
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM7183

(3-(4-methylphenyl)-3,4,10,11-tetraazatricyclo[7.3....)Show InChI InChI=1S/C16H13N5O/c1-9-2-4-10(5-3-9)21-15-11(14(20-21)16(17)22)6-7-13-12(15)8-18-19-13/h2-8H,1H3,(H2,17,22)(H,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence ATP/[gamma-33P] ATP. After in... |
Bioorg Med Chem Lett 15: 1315-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.01.023
BindingDB Entry DOI: 10.7270/Q2N58JKH |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50435043
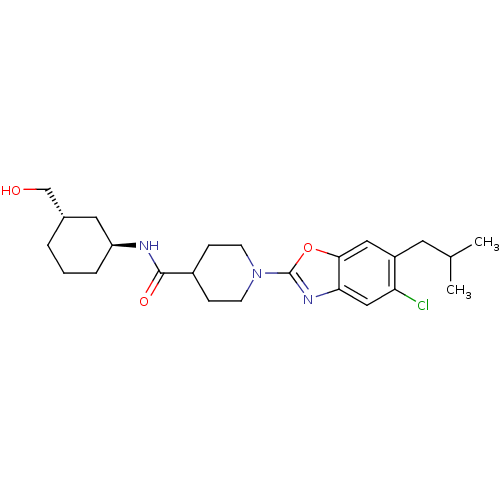
(CHEMBL2391296)Show SMILES CC(C)Cc1cc2oc(nc2cc1Cl)N1CCC(CC1)C(=O)N[C@H]1CCC[C@H](CO)C1 |r| Show InChI InChI=1S/C24H34ClN3O3/c1-15(2)10-18-12-22-21(13-20(18)25)27-24(31-22)28-8-6-17(7-9-28)23(30)26-19-5-3-4-16(11-19)14-29/h12-13,15-17,19,29H,3-11,14H2,1-2H3,(H,26,30)/t16-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mPGES-1 (unknown origin) using PGH2 as substrate assessed as PGE2 synthesis by ELISA |
Bioorg Med Chem Lett 23: 1114-9 (2013)
Article DOI: 10.1016/j.bmcl.2012.11.109
BindingDB Entry DOI: 10.7270/Q2FQ9Z0W |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50435042
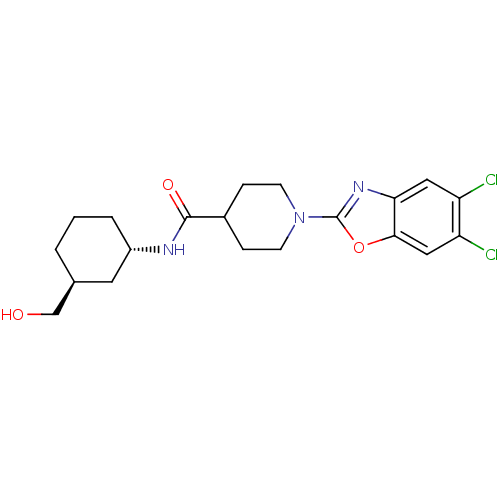
(CHEMBL2391297)Show SMILES OC[C@H]1CCC[C@@H](C1)NC(=O)C1CCN(CC1)c1nc2cc(Cl)c(Cl)cc2o1 |r| Show InChI InChI=1S/C20H25Cl2N3O3/c21-15-9-17-18(10-16(15)22)28-20(24-17)25-6-4-13(5-7-25)19(27)23-14-3-1-2-12(8-14)11-26/h9-10,12-14,26H,1-8,11H2,(H,23,27)/t12-,14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mPGES-1 (unknown origin) using PGH2 as substrate assessed as PGE2 synthesis by ELISA |
Bioorg Med Chem Lett 23: 1114-9 (2013)
Article DOI: 10.1016/j.bmcl.2012.11.109
BindingDB Entry DOI: 10.7270/Q2FQ9Z0W |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50426955
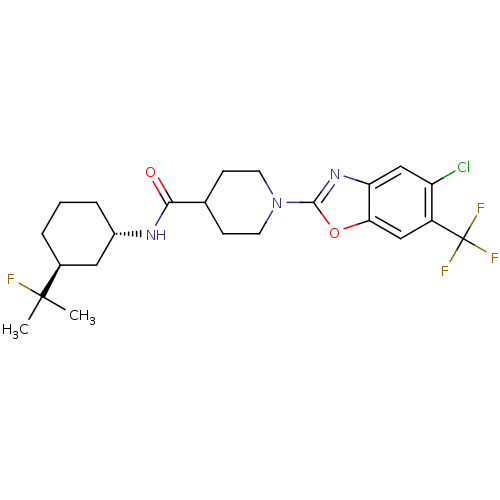
(CHEMBL2325077)Show SMILES CC(C)(F)[C@H]1CCC[C@@H](C1)NC(=O)C1CCN(CC1)c1nc2cc(Cl)c(cc2o1)C(F)(F)F |r| Show InChI InChI=1S/C23H28ClF4N3O2/c1-22(2,25)14-4-3-5-15(10-14)29-20(32)13-6-8-31(9-7-13)21-30-18-12-17(24)16(23(26,27)28)11-19(18)33-21/h11-15H,3-10H2,1-2H3,(H,29,32)/t14-,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human mPGES1 by ELISA |
Bioorg Med Chem Lett 23: 1120-6 (2013)
Article DOI: 10.1016/j.bmcl.2012.11.107
BindingDB Entry DOI: 10.7270/Q26W9CDS |
More data for this
Ligand-Target Pair | |
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM7212
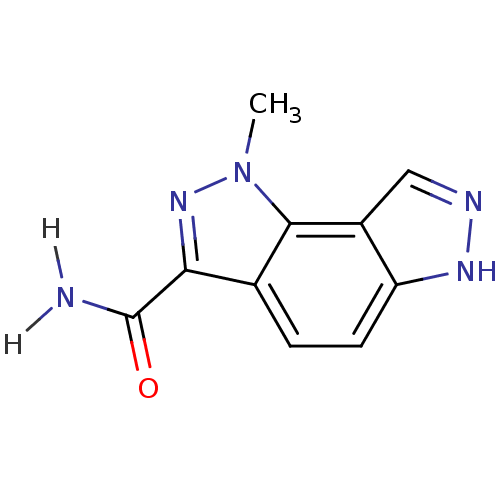
(3-methyl-3,4,10,11-tetraazatricyclo[7.3.0.0^{2,6}]...)Show InChI InChI=1S/C10H9N5O/c1-15-9-5(8(14-15)10(11)16)2-3-7-6(9)4-12-13-7/h2-4H,1H3,(H2,11,16)(H,12,13) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence ATP/[gamma-33P] ATP. After in... |
Bioorg Med Chem Lett 15: 1315-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.01.023
BindingDB Entry DOI: 10.7270/Q2N58JKH |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM7197

(3-[4-(morpholin-4-yl)phenyl]-3,4,10,11-tetraazatri...)Show SMILES NC(=O)c1nn(-c2ccc(cc2)N2CCOCC2)c2c1ccc1[nH]ncc21 Show InChI InChI=1S/C19H18N6O2/c20-19(26)17-14-5-6-16-15(11-21-22-16)18(14)25(23-17)13-3-1-12(2-4-13)24-7-9-27-10-8-24/h1-6,11H,7-10H2,(H2,20,26)(H,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence ATP/[gamma-33P] ATP. After in... |
Bioorg Med Chem Lett 15: 1315-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.01.023
BindingDB Entry DOI: 10.7270/Q2N58JKH |
More data for this
Ligand-Target Pair | |
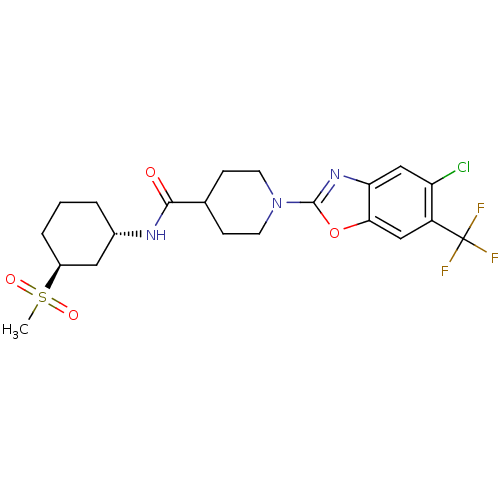
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50426954
(CHEMBL2325078)Show SMILES CS(=O)(=O)[C@H]1CCC[C@@H](C1)NC(=O)C1CCN(CC1)c1nc2cc(Cl)c(cc2o1)C(F)(F)F |r| Show InChI InChI=1S/C21H25ClF3N3O4S/c1-33(30,31)14-4-2-3-13(9-14)26-19(29)12-5-7-28(8-6-12)20-27-17-11-16(22)15(21(23,24)25)10-18(17)32-20/h10-14H,2-9H2,1H3,(H,26,29)/t13-,14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human mPGES1 by ELISA |
Bioorg Med Chem Lett 23: 1120-6 (2013)
Article DOI: 10.1016/j.bmcl.2012.11.107
BindingDB Entry DOI: 10.7270/Q26W9CDS |
More data for this
Ligand-Target Pair | |
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM7215

(3-(2,2,2-trifluoroethyl)-3,4,10,11-tetraazatricycl...)Show InChI InChI=1S/C11H10F3N5O/c12-11(13,14)4-19-9-5(8(18-19)10(15)20)1-2-7-6(9)3-16-17-7/h3H,1-2,4H2,(H2,15,20)(H,16,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence ATP/[gamma-33P] ATP. After in... |
Bioorg Med Chem Lett 15: 1315-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.01.023
BindingDB Entry DOI: 10.7270/Q2N58JKH |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50435041
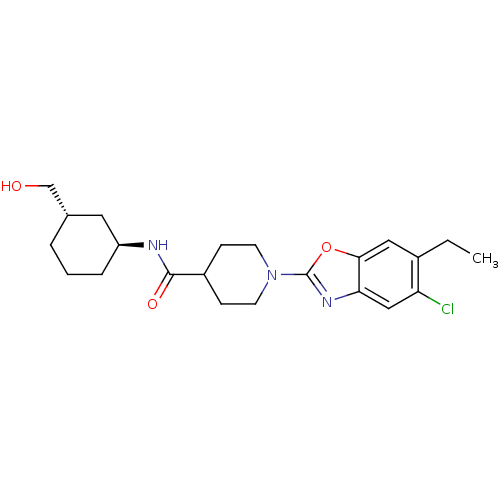
(CHEMBL2391294)Show SMILES CCc1cc2oc(nc2cc1Cl)N1CCC(CC1)C(=O)N[C@H]1CCC[C@H](CO)C1 |r| Show InChI InChI=1S/C22H30ClN3O3/c1-2-15-11-20-19(12-18(15)23)25-22(29-20)26-8-6-16(7-9-26)21(28)24-17-5-3-4-14(10-17)13-27/h11-12,14,16-17,27H,2-10,13H2,1H3,(H,24,28)/t14-,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mPGES-1 (unknown origin) using PGH2 as substrate assessed as PGE2 synthesis by ELISA |
Bioorg Med Chem Lett 23: 1114-9 (2013)
Article DOI: 10.1016/j.bmcl.2012.11.109
BindingDB Entry DOI: 10.7270/Q2FQ9Z0W |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50426963
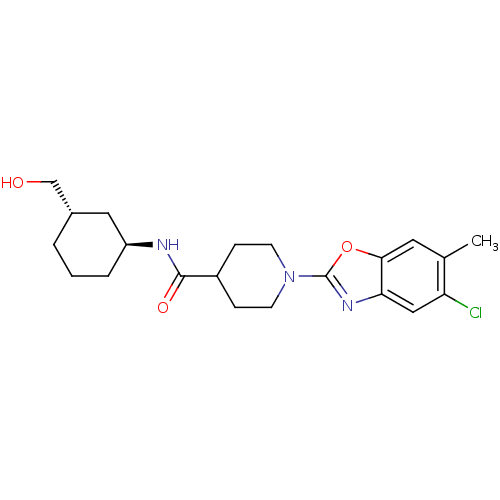
(CHEMBL2325084)Show SMILES Cc1cc2oc(nc2cc1Cl)N1CCC(CC1)C(=O)N[C@H]1CCC[C@H](CO)C1 |r| Show InChI InChI=1S/C21H28ClN3O3/c1-13-9-19-18(11-17(13)22)24-21(28-19)25-7-5-15(6-8-25)20(27)23-16-4-2-3-14(10-16)12-26/h9,11,14-16,26H,2-8,10,12H2,1H3,(H,23,27)/t14-,16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human mPGES1 by ELISA |
Bioorg Med Chem Lett 23: 1120-6 (2013)
Article DOI: 10.1016/j.bmcl.2012.11.107
BindingDB Entry DOI: 10.7270/Q26W9CDS |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50426963

(CHEMBL2325084)Show SMILES Cc1cc2oc(nc2cc1Cl)N1CCC(CC1)C(=O)N[C@H]1CCC[C@H](CO)C1 |r| Show InChI InChI=1S/C21H28ClN3O3/c1-13-9-19-18(11-17(13)22)24-21(28-19)25-7-5-15(6-8-25)20(27)23-16-4-2-3-14(10-16)12-26/h9,11,14-16,26H,2-8,10,12H2,1H3,(H,23,27)/t14-,16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mPGES-1 (unknown origin) using PGH2 as substrate assessed as PGE2 synthesis by ELISA |
Bioorg Med Chem Lett 23: 1114-9 (2013)
Article DOI: 10.1016/j.bmcl.2012.11.109
BindingDB Entry DOI: 10.7270/Q2FQ9Z0W |
More data for this
Ligand-Target Pair | |
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM7204

(3-(3-fluorophenyl)-3,4,10,11-tetraazatricyclo[7.3....)Show InChI InChI=1S/C15H10FN5O/c16-8-2-1-3-9(6-8)21-14-10(13(20-21)15(17)22)4-5-12-11(14)7-18-19-12/h1-7H,(H2,17,22)(H,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence ATP/[gamma-33P] ATP. After in... |
Bioorg Med Chem Lett 15: 1315-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.01.023
BindingDB Entry DOI: 10.7270/Q2N58JKH |
More data for this
Ligand-Target Pair | |
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM7216

(3-(2-hydroxyethyl)-3,4,10,11-tetraazatricyclo[7.3....)Show InChI InChI=1S/C11H11N5O2/c12-11(18)9-6-1-2-8-7(5-13-14-8)10(6)16(15-9)3-4-17/h1-2,5,17H,3-4H2,(H2,12,18)(H,13,14) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence ATP/[gamma-33P] ATP. After in... |
Bioorg Med Chem Lett 15: 1315-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.01.023
BindingDB Entry DOI: 10.7270/Q2N58JKH |
More data for this
Ligand-Target Pair | |
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM7224

(N-hydroxy-3-(4-methoxyphenyl)-3,4,10,11-tetraazatr...)Show SMILES COc1ccc(cc1)-n1nc(C(=O)NO)c2ccc3[nH]ncc3c12 Show InChI InChI=1S/C16H13N5O3/c1-24-10-4-2-9(3-5-10)21-15-11(14(19-21)16(22)20-23)6-7-13-12(15)8-17-18-13/h2-8,23H,1H3,(H,17,18)(H,20,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence ATP/[gamma-33P] ATP. After in... |
Bioorg Med Chem Lett 15: 1315-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.01.023
BindingDB Entry DOI: 10.7270/Q2N58JKH |
More data for this
Ligand-Target Pair | |
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM7202

(3-(3-chlorophenyl)-3,4,10,11-tetraazatricyclo[7.3....)Show InChI InChI=1S/C15H10ClN5O/c16-8-2-1-3-9(6-8)21-14-10(13(20-21)15(17)22)4-5-12-11(14)7-18-19-12/h1-7H,(H2,17,22)(H,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence ATP/[gamma-33P] ATP. After in... |
Bioorg Med Chem Lett 15: 1315-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.01.023
BindingDB Entry DOI: 10.7270/Q2N58JKH |
More data for this
Ligand-Target Pair | |
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM7176

(3-(4-methanesulfonylphenyl)-3,4,10,11-tetraazatric...)Show SMILES CS(=O)(=O)c1ccc(cc1)-n1nc(C(N)=O)c2CCc3[nH]ncc3-c12 Show InChI InChI=1S/C16H15N5O3S/c1-25(23,24)10-4-2-9(3-5-10)21-15-11(14(20-21)16(17)22)6-7-13-12(15)8-18-19-13/h2-5,8H,6-7H2,1H3,(H2,17,22)(H,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Nerviano Medical Sciences
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence ATP/[gamma-33P] ATP. After in... |
Bioorg Med Chem Lett 15: 1315-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.01.023
BindingDB Entry DOI: 10.7270/Q2N58JKH |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50426957
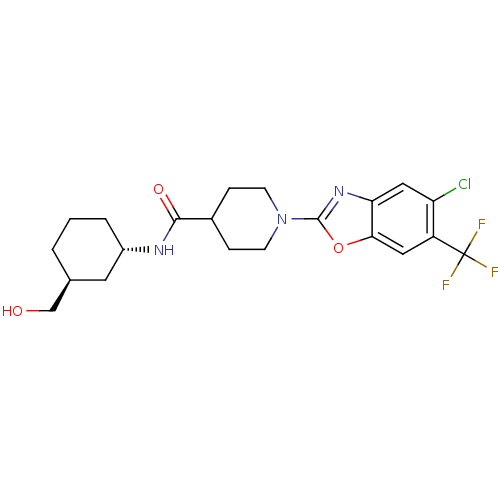
(CHEMBL2325075)Show SMILES OC[C@H]1CCC[C@@H](C1)NC(=O)C1CCN(CC1)c1nc2cc(Cl)c(cc2o1)C(F)(F)F |r| Show InChI InChI=1S/C21H25ClF3N3O3/c22-16-10-17-18(9-15(16)21(23,24)25)31-20(27-17)28-6-4-13(5-7-28)19(30)26-14-3-1-2-12(8-14)11-29/h9-10,12-14,29H,1-8,11H2,(H,26,30)/t12-,14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human mPGES1 by ELISA |
Bioorg Med Chem Lett 23: 1120-6 (2013)
Article DOI: 10.1016/j.bmcl.2012.11.107
BindingDB Entry DOI: 10.7270/Q26W9CDS |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50426957

(CHEMBL2325075)Show SMILES OC[C@H]1CCC[C@@H](C1)NC(=O)C1CCN(CC1)c1nc2cc(Cl)c(cc2o1)C(F)(F)F |r| Show InChI InChI=1S/C21H25ClF3N3O3/c22-16-10-17-18(9-15(16)21(23,24)25)31-20(27-17)28-6-4-13(5-7-28)19(30)26-14-3-1-2-12(8-14)11-29/h9-10,12-14,29H,1-8,11H2,(H,26,30)/t12-,14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mPGES-1 (unknown origin) using PGH2 as substrate assessed as PGE2 synthesis by ELISA |
Bioorg Med Chem Lett 23: 1114-9 (2013)
Article DOI: 10.1016/j.bmcl.2012.11.109
BindingDB Entry DOI: 10.7270/Q2FQ9Z0W |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50426960

(CHEMBL2325072)Show SMILES Cc1cc2oc(nc2cc1Cl)N1CCC(CC1)C(=O)N[C@H]1CC[C@H](CC1)C(C)(C)O |r,wU:20.22,23.29,(3.97,-46.23,;5.31,-45.46,;6.64,-46.23,;7.97,-45.46,;9.45,-45.94,;10.36,-44.69,;9.45,-43.43,;7.97,-43.91,;6.64,-43.15,;5.31,-43.92,;3.98,-43.15,;11.9,-44.69,;12.66,-46.03,;14.19,-46.03,;14.97,-44.7,;14.2,-43.37,;12.66,-43.36,;16.51,-44.71,;17.28,-43.38,;17.27,-46.04,;18.81,-46.05,;19.58,-44.73,;21.13,-44.74,;21.89,-46.08,;21.11,-47.41,;19.57,-47.4,;23.43,-46.09,;24.21,-44.77,;24.97,-46.09,;24.19,-47.43,)| Show InChI InChI=1S/C23H32ClN3O3/c1-14-12-20-19(13-18(14)24)26-22(30-20)27-10-8-15(9-11-27)21(28)25-17-6-4-16(5-7-17)23(2,3)29/h12-13,15-17,29H,4-11H2,1-3H3,(H,25,28)/t16-,17+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human mPGES1 by ELISA |
Bioorg Med Chem Lett 23: 1120-6 (2013)
Article DOI: 10.1016/j.bmcl.2012.11.107
BindingDB Entry DOI: 10.7270/Q26W9CDS |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50425318
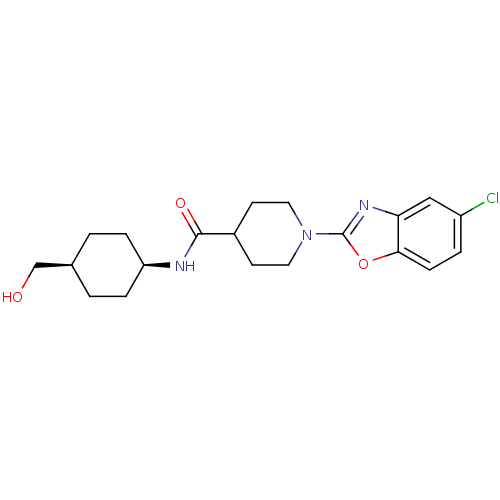
(CHEMBL2315861)Show SMILES OC[C@H]1CC[C@H](CC1)NC(=O)C1CCN(CC1)c1nc2cc(Cl)ccc2o1 |r,wD:5.8,2.1,(21.18,-14.42,;20.41,-13.09,;18.87,-13.08,;18.1,-11.75,;16.56,-11.75,;15.8,-13.08,;16.57,-14.42,;18.1,-14.42,;14.27,-13.08,;13.49,-11.75,;14.26,-10.42,;11.95,-11.75,;11.18,-13.09,;9.65,-13.09,;8.88,-11.77,;9.63,-10.43,;11.18,-10.42,;7.34,-11.77,;6.44,-13.03,;4.97,-12.56,;3.63,-13.34,;2.3,-12.57,;.96,-13.34,;2.3,-11.02,;3.63,-10.25,;4.96,-11.02,;6.43,-10.53,)| Show InChI InChI=1S/C20H26ClN3O3/c21-15-3-6-18-17(11-15)23-20(27-18)24-9-7-14(8-10-24)19(26)22-16-4-1-13(12-25)2-5-16/h3,6,11,13-14,16,25H,1-2,4-5,7-10,12H2,(H,22,26)/t13-,16+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mPGES-1-mediated PGE2 production in LPS-stimulated healthy human whole blood after 20 to 24 hrs by ELISA |
Bioorg Med Chem Lett 23: 1114-9 (2013)
Article DOI: 10.1016/j.bmcl.2012.11.109
BindingDB Entry DOI: 10.7270/Q2FQ9Z0W |
More data for this
Ligand-Target Pair | |
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM7189

(3-(4-fluorophenyl)-3,4,10,11-tetraazatricyclo[7.3....)Show InChI InChI=1S/C15H10FN5O/c16-8-1-3-9(4-2-8)21-14-10(13(20-21)15(17)22)5-6-12-11(14)7-18-19-12/h1-7H,(H2,17,22)(H,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence ATP/[gamma-33P] ATP. After in... |
Bioorg Med Chem Lett 15: 1315-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.01.023
BindingDB Entry DOI: 10.7270/Q2N58JKH |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM7193

(3-[4-(trifluoromethoxy)phenyl]-3,4,10,11-tetraazat...)Show SMILES NC(=O)c1nn(-c2ccc(OC(F)(F)F)cc2)c2c1ccc1[nH]ncc21 Show InChI InChI=1S/C16H10F3N5O2/c17-16(18,19)26-9-3-1-8(2-4-9)24-14-10(13(23-24)15(20)25)5-6-12-11(14)7-21-22-12/h1-7H,(H2,20,25)(H,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence ATP/[gamma-33P] ATP. After in... |
Bioorg Med Chem Lett 15: 1315-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.01.023
BindingDB Entry DOI: 10.7270/Q2N58JKH |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50426964
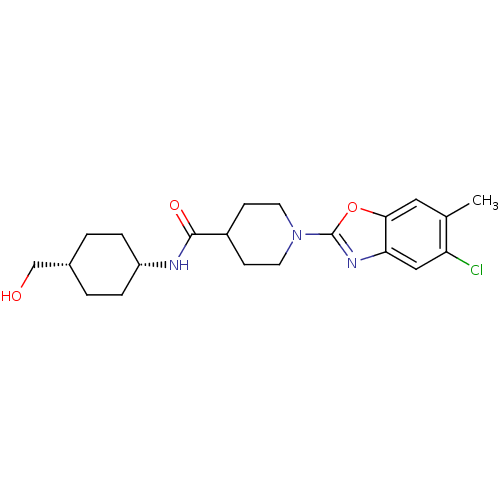
(CHEMBL2325083)Show SMILES Cc1cc2oc(nc2cc1Cl)N1CCC(CC1)C(=O)N[C@@H]1CC[C@H](CO)CC1 |r,wU:20.22,23.26,(25.8,-26.07,;27.13,-25.3,;28.47,-26.07,;29.8,-25.3,;31.27,-25.78,;32.19,-24.53,;31.27,-23.27,;29.8,-23.75,;28.46,-22.99,;27.13,-23.76,;25.8,-22.99,;33.72,-24.53,;34.48,-25.87,;36.01,-25.87,;36.79,-24.54,;36.02,-23.21,;34.48,-23.2,;38.33,-24.55,;39.1,-23.22,;39.09,-25.88,;40.63,-25.89,;41.4,-27.24,;42.93,-27.25,;43.71,-25.92,;45.25,-25.93,;46.01,-27.27,;42.95,-24.58,;41.41,-24.57,)| Show InChI InChI=1S/C21H28ClN3O3/c1-13-10-19-18(11-17(13)22)24-21(28-19)25-8-6-15(7-9-25)20(27)23-16-4-2-14(12-26)3-5-16/h10-11,14-16,26H,2-9,12H2,1H3,(H,23,27)/t14-,16+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human mPGES1 by ELISA |
Bioorg Med Chem Lett 23: 1120-6 (2013)
Article DOI: 10.1016/j.bmcl.2012.11.107
BindingDB Entry DOI: 10.7270/Q26W9CDS |
More data for this
Ligand-Target Pair | |
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM7168
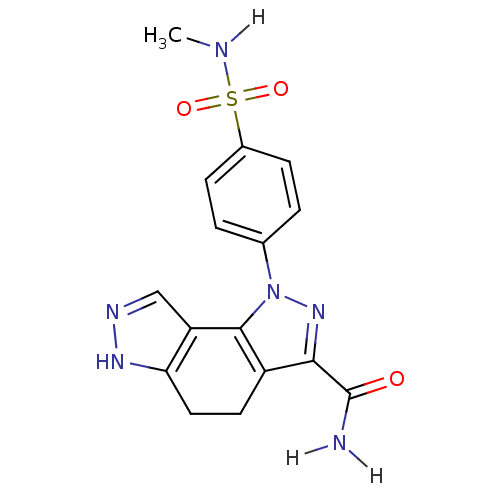
(3-[4-(methylsulfamoyl)phenyl]-3,4,10,11-tetraazatr...)Show SMILES CNS(=O)(=O)c1ccc(cc1)-n1nc(C(N)=O)c2CCc3[nH]ncc3-c12 Show InChI InChI=1S/C16H16N6O3S/c1-18-26(24,25)10-4-2-9(3-5-10)22-15-11(14(21-22)16(17)23)6-7-13-12(15)8-19-20-13/h2-5,8,18H,6-7H2,1H3,(H2,17,23)(H,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Nerviano Medical Sciences
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence ATP/[gamma-33P] ATP. After in... |
Bioorg Med Chem Lett 15: 1315-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.01.023
BindingDB Entry DOI: 10.7270/Q2N58JKH |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50435054
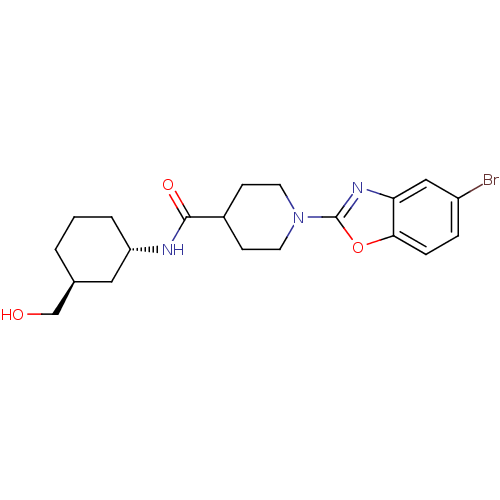
(CHEMBL2391289)Show SMILES OC[C@H]1CCC[C@@H](C1)NC(=O)C1CCN(CC1)c1nc2cc(Br)ccc2o1 |r| Show InChI InChI=1S/C20H26BrN3O3/c21-15-4-5-18-17(11-15)23-20(27-18)24-8-6-14(7-9-24)19(26)22-16-3-1-2-13(10-16)12-25/h4-5,11,13-14,16,25H,1-3,6-10,12H2,(H,22,26)/t13-,16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mPGES-1 (unknown origin) using PGH2 as substrate assessed as PGE2 synthesis by ELISA |
Bioorg Med Chem Lett 23: 1114-9 (2013)
Article DOI: 10.1016/j.bmcl.2012.11.109
BindingDB Entry DOI: 10.7270/Q2FQ9Z0W |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data