| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Lysine-specific histone demethylase 1A |
|---|
| Ligand | BDBM50446142 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_1288151 (CHEMBL3110654) |
|---|
| Ki | 120±n/a nM |
|---|
| Citation |  Kumarasinghe, IR; Woster, PM Synthesis and evaluation of novel cyclic Peptide inhibitors of lysine-specific demethylase 1. ACS Med Chem Lett5:29-33 (2014) [PubMed] Article Kumarasinghe, IR; Woster, PM Synthesis and evaluation of novel cyclic Peptide inhibitors of lysine-specific demethylase 1. ACS Med Chem Lett5:29-33 (2014) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Lysine-specific histone demethylase 1A |
|---|
| Name: | Lysine-specific histone demethylase 1A |
|---|
| Synonyms: | AOF2 | BRAF35-HDAC complex protein BHC110 | Flavin-containing amine oxidase domain-containing protein 2 | KDM1 | KDM1A | KDM1A_HUMAN | KIAA0601 | LSD1 | Lysine-specific demethylase 1 (LSD1) | Lysine-specific histone demethylase 1 | Lysine-specific histone demethylase 1 (LSD1) |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 92901.01 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | O60341 |
|---|
| Residue: | 852 |
|---|
| Sequence: | MLSGKKAAAAAAAAAAAATGTEAGPGTAGGSENGSEVAAQPAGLSGPAEVGPGAVGERTP
RKKEPPRASPPGGLAEPPGSAGPQAGPTVVPGSATPMETGIAETPEGRRTSRRKRAKVEY
REMDESLANLSEDEYYSEEERNAKAEKEKKLPPPPPQAPPEEENESEPEEPSGVEGAAFQ
SRLPHDRMTSQEAACFPDIISGPQQTQKVFLFIRNRTLQLWLDNPKIQLTFEATLQQLEA
PYNSDTVLVHRVHSYLERHGLINFGIYKRIKPLPTKKTGKVIIIGSGVSGLAAARQLQSF
GMDVTLLEARDRVGGRVATFRKGNYVADLGAMVVTGLGGNPMAVVSKQVNMELAKIKQKC
PLYEANGQAVPKEKDEMVEQEFNRLLEATSYLSHQLDFNVLNNKPVSLGQALEVVIQLQE
KHVKDEQIEHWKKIVKTQEELKELLNKMVNLKEKIKELHQQYKEASEVKPPRDITAEFLV
KSKHRDLTALCKEYDELAETQGKLEEKLQELEANPPSDVYLSSRDRQILDWHFANLEFAN
ATPLSTLSLKHWDQDDDFEFTGSHLTVRNGYSCVPVALAEGLDIKLNTAVRQVRYTASGC
EVIAVNTRSTSQTFIYKCDAVLCTLPLGVLKQQPPAVQFVPPLPEWKTSAVQRMGFGNLN
KVVLCFDRVFWDPSVNLFGHVGSTTASRGELFLFWNLYKAPILLALVAGEAAGIMENISD
DVIVGRCLAILKGIFGSSAVPQPKETVVSRWRADPWARGSYSYVAAGSSGNDYDLMAQPI
TPGPSIPGAPQPIPRLFFAGEHTIRNYPATVHGALLSGLREAGRIADQFLGAMYTLPRQA
TPGVPAQQSPSM
|
|
|
|---|
| BDBM50446142 |
|---|
| n/a |
|---|
| Name | BDBM50446142 |
|---|
| Synonyms: | CHEMBL3108892 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C103H180N36O28 |
|---|
| Mol. Mass. | 2370.7557 |
|---|
| SMILES | [#6]-[#6](-[#6])-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6]-[#7]-1-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7]-[#6@@H]-1-[#6]-[#6@H]-1-c1ccccc1)-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#7]-[#6](-[#7])=[#7])-[#7]-[#6](=O)-[#6@H](-[#6])-[#7])-[#6@@H](-[#6])-[#8])-[#6@@H](-[#6])-[#8])-[#6@@H](-[#6])-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](-[#8])=O |r| |
|---|
| Structure | 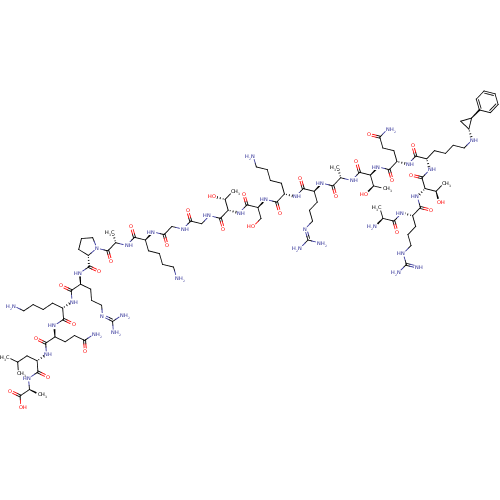
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Kumarasinghe, IR; Woster, PM Synthesis and evaluation of novel cyclic Peptide inhibitors of lysine-specific demethylase 1. ACS Med Chem Lett5:29-33 (2014) [PubMed] Article
Kumarasinghe, IR; Woster, PM Synthesis and evaluation of novel cyclic Peptide inhibitors of lysine-specific demethylase 1. ACS Med Chem Lett5:29-33 (2014) [PubMed] Article