| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Cytochrome P450 2D6 |
|---|
| Ligand | BDBM50446472 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_1287805 (CHEMBL3111800) |
|---|
| IC50 | 2700±n/a nM |
|---|
| Citation |  Bürli, RW; Luckhurst, CA; Aziz, O; Matthews, KL; Yates, D; Lyons, KA; Beconi, M; McAllister, G; Breccia, P; Stott, AJ; Penrose, SD; Wall, M; Lamers, M; Leonard, P; Müller, I; Richardson, CM; Jarvis, R; Stones, L; Hughes, S; Wishart, G; Haughan, AF; O'Connell, C; Mead, T; McNeil, H; Vann, J; Mangette, J; Maillard, M; Beaumont, V; Munoz-Sanjuan, I; Dominguez, C Design, synthesis, and biological evaluation of potent and selective class IIa histone deacetylase (HDAC) inhibitors as a potential therapy for Huntington's disease. J Med Chem56:9934-54 (2013) [PubMed] Article Bürli, RW; Luckhurst, CA; Aziz, O; Matthews, KL; Yates, D; Lyons, KA; Beconi, M; McAllister, G; Breccia, P; Stott, AJ; Penrose, SD; Wall, M; Lamers, M; Leonard, P; Müller, I; Richardson, CM; Jarvis, R; Stones, L; Hughes, S; Wishart, G; Haughan, AF; O'Connell, C; Mead, T; McNeil, H; Vann, J; Mangette, J; Maillard, M; Beaumont, V; Munoz-Sanjuan, I; Dominguez, C Design, synthesis, and biological evaluation of potent and selective class IIa histone deacetylase (HDAC) inhibitors as a potential therapy for Huntington's disease. J Med Chem56:9934-54 (2013) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Cytochrome P450 2D6 |
|---|
| Name: | Cytochrome P450 2D6 |
|---|
| Synonyms: | CP2D6_HUMAN | CYP2D6 | CYP2DL1 | CYPIID6 | Cytochrome P450 2D6 (CYP2D6) | Debrisoquine 4-hydroxylase | P450-DB1 |
|---|
| Type: | Protein |
|---|
| Mol. Mass.: | 55774.82 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P10635 |
|---|
| Residue: | 497 |
|---|
| Sequence: | MGLEALVPLAVIVAIFLLLVDLMHRRQRWAARYPPGPLPLPGLGNLLHVDFQNTPYCFDQ
LRRRFGDVFSLQLAWTPVVVLNGLAAVREALVTHGEDTADRPPVPITQILGFGPRSQGVF
LARYGPAWREQRRFSVSTLRNLGLGKKSLEQWVTEEAACLCAAFANHSGRPFRPNGLLDK
AVSNVIASLTCGRRFEYDDPRFLRLLDLAQEGLKEESGFLREVLNAVPVLLHIPALAGKV
LRFQKAFLTQLDELLTEHRMTWDPAQPPRDLTEAFLAEMEKAKGNPESSFNDENLRIVVA
DLFSAGMVTTSTTLAWGLLLMILHPDVQRRVQQEIDDVIGQVRRPEMGDQAHMPYTTAVI
HEVQRFGDIVPLGVTHMTSRDIEVQGFRIPKGTTLITNLSSVLKDEAVWEKPFRFHPEHF
LDAQGHFVKPEAFLPFSAGRRACLGEPLARMELFLFFTSLLQHFSFSVPTGQPRPSHHGV
FAFLVSPSPYELCAVPR
|
|
|
|---|
| BDBM50446472 |
|---|
| n/a |
|---|
| Name | BDBM50446472 |
|---|
| Synonyms: | CHEMBL3110021 | US9765054, Compound 41B |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C19H16N2O3 |
|---|
| Mol. Mass. | 320.3419 |
|---|
| SMILES | ONC(=O)[C@@H]1[C@@H]([C@H]1c1ccc(cc1)-c1cnco1)c1ccccc1 |r| |
|---|
| Structure | 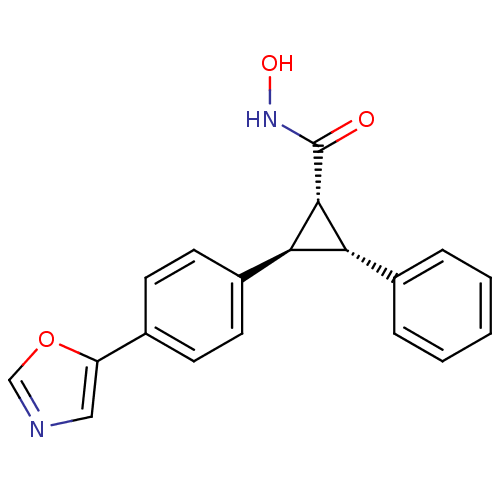
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Bürli, RW; Luckhurst, CA; Aziz, O; Matthews, KL; Yates, D; Lyons, KA; Beconi, M; McAllister, G; Breccia, P; Stott, AJ; Penrose, SD; Wall, M; Lamers, M; Leonard, P; Müller, I; Richardson, CM; Jarvis, R; Stones, L; Hughes, S; Wishart, G; Haughan, AF; O'Connell, C; Mead, T; McNeil, H; Vann, J; Mangette, J; Maillard, M; Beaumont, V; Munoz-Sanjuan, I; Dominguez, C Design, synthesis, and biological evaluation of potent and selective class IIa histone deacetylase (HDAC) inhibitors as a potential therapy for Huntington's disease. J Med Chem56:9934-54 (2013) [PubMed] Article
Bürli, RW; Luckhurst, CA; Aziz, O; Matthews, KL; Yates, D; Lyons, KA; Beconi, M; McAllister, G; Breccia, P; Stott, AJ; Penrose, SD; Wall, M; Lamers, M; Leonard, P; Müller, I; Richardson, CM; Jarvis, R; Stones, L; Hughes, S; Wishart, G; Haughan, AF; O'Connell, C; Mead, T; McNeil, H; Vann, J; Mangette, J; Maillard, M; Beaumont, V; Munoz-Sanjuan, I; Dominguez, C Design, synthesis, and biological evaluation of potent and selective class IIa histone deacetylase (HDAC) inhibitors as a potential therapy for Huntington's disease. J Med Chem56:9934-54 (2013) [PubMed] Article