| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Cholinesterase |
|---|
| Ligand | BDBM50448131 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_1455579 (CHEMBL3364956) |
|---|
| IC50 | 920±n/a nM |
|---|
| Citation |  Di Pietro, O; Pérez-Areales, FJ; Juárez-Jiménez, J; Espargaró, A; Clos, MV; Pérez, B; Lavilla, R; Sabaté, R; Luque, FJ; Muñoz-Torrero, D Tetrahydrobenzo[h][1,6]naphthyridine-6-chlorotacrine hybrids as a new family of anti-Alzheimer agents targetingß-amyloid, tau, and cholinesterase pathologies. Eur J Med Chem84:107-17 (2014) [PubMed] Article Di Pietro, O; Pérez-Areales, FJ; Juárez-Jiménez, J; Espargaró, A; Clos, MV; Pérez, B; Lavilla, R; Sabaté, R; Luque, FJ; Muñoz-Torrero, D Tetrahydrobenzo[h][1,6]naphthyridine-6-chlorotacrine hybrids as a new family of anti-Alzheimer agents targetingß-amyloid, tau, and cholinesterase pathologies. Eur J Med Chem84:107-17 (2014) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Cholinesterase |
|---|
| Name: | Cholinesterase |
|---|
| Synonyms: | Acylcholine acylhydrolase | BCHE | Butyrylcholine esterase (BChE) | Butyrylcholinesterase (BChE) | Butyrylcholinesterase (BuChE) | CHE1 | CHLE_HUMAN | Choline esterase II | Cholinesterases | Cholinesterases; ACHE & BCHE | Pseudocholinesterase |
|---|
| Type: | Homotetramer |
|---|
| Mol. Mass.: | 68422.27 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P06276 |
|---|
| Residue: | 602 |
|---|
| Sequence: | MHSKVTIICIRFLFWFLLLCMLIGKSHTEDDIIIATKNGKVRGMNLTVFGGTVTAFLGIP
YAQPPLGRLRFKKPQSLTKWSDIWNATKYANSCCQNIDQSFPGFHGSEMWNPNTDLSEDC
LYLNVWIPAPKPKNATVLIWIYGGGFQTGTSSLHVYDGKFLARVERVIVVSMNYRVGALG
FLALPGNPEAPGNMGLFDQQLALQWVQKNIAAFGGNPKSVTLFGESAGAASVSLHLLSPG
SHSLFTRAILQSGSFNAPWAVTSLYEARNRTLNLAKLTGCSRENETEIIKCLRNKDPQEI
LLNEAFVVPYGTPLSVNFGPTVDGDFLTDMPDILLELGQFKKTQILVGVNKDEGTAFLVY
GAPGFSKDNNSIITRKEFQEGLKIFFPGVSEFGKESILFHYTDWVDDQRPENYREALGDV
VGDYNFICPALEFTKKFSEWGNNAFFYYFEHRSSKLPWPEWMGVMHGYEIEFVFGLPLER
RDNYTKAEEILSRSIVKRWANFAKYGNPNETQNNSTSWPVFKSTEQKYLTLNTESTRIMT
KLRAQQCRFWTSFFPKVLEMTGNIDEAEWEWKAGFHRWNNYMMDWKNQFNDYTSKKESCV
GL
|
|
|
|---|
| BDBM50448131 |
|---|
| n/a |
|---|
| Name | BDBM50448131 |
|---|
| Synonyms: | CHEMBL3122167 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C21H20ClN3O |
|---|
| Mol. Mass. | 365.856 |
|---|
| SMILES | CCNC(=O)c1ccc2nc(-c3ccc(Cl)cc3)c3CCCNc3c2c1 |
|---|
| Structure | 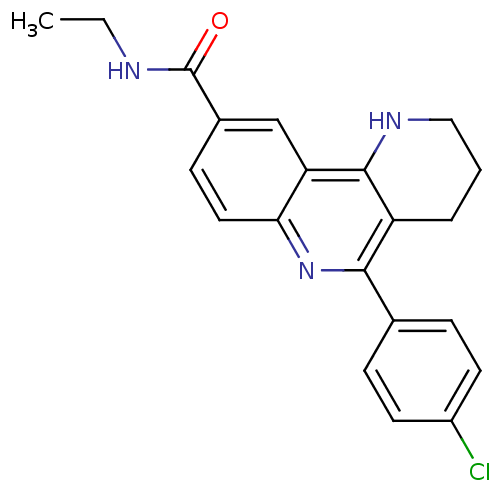
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Di Pietro, O; Pérez-Areales, FJ; Juárez-Jiménez, J; Espargaró, A; Clos, MV; Pérez, B; Lavilla, R; Sabaté, R; Luque, FJ; Muñoz-Torrero, D Tetrahydrobenzo[h][1,6]naphthyridine-6-chlorotacrine hybrids as a new family of anti-Alzheimer agents targetingß-amyloid, tau, and cholinesterase pathologies. Eur J Med Chem84:107-17 (2014) [PubMed] Article
Di Pietro, O; Pérez-Areales, FJ; Juárez-Jiménez, J; Espargaró, A; Clos, MV; Pérez, B; Lavilla, R; Sabaté, R; Luque, FJ; Muñoz-Torrero, D Tetrahydrobenzo[h][1,6]naphthyridine-6-chlorotacrine hybrids as a new family of anti-Alzheimer agents targetingß-amyloid, tau, and cholinesterase pathologies. Eur J Med Chem84:107-17 (2014) [PubMed] Article