

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50049862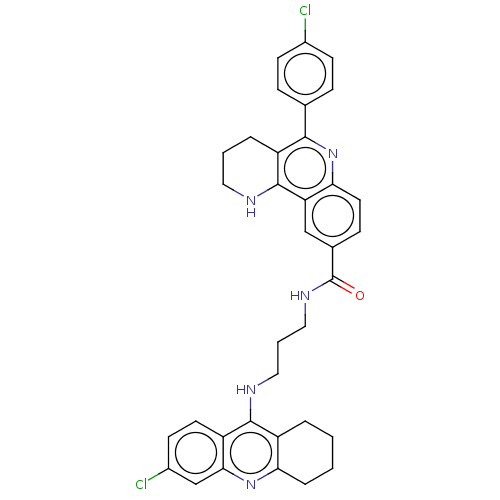 (CHEMBL3322232) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate by spectrophotometric analysis | Eur J Med Chem 84: 107-17 (2014) Article DOI: 10.1016/j.ejmech.2014.07.021 BindingDB Entry DOI: 10.7270/Q2571DNF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10592 (7-chloro-15-methyl-10-azatetracyclo[11.3.1.0^{2,11...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 20 mins before substrate addition by Ellman method | Bioorg Med Chem 22: 5298-307 (2014) Article DOI: 10.1016/j.bmc.2014.07.053 BindingDB Entry DOI: 10.7270/Q2M61MV9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50049859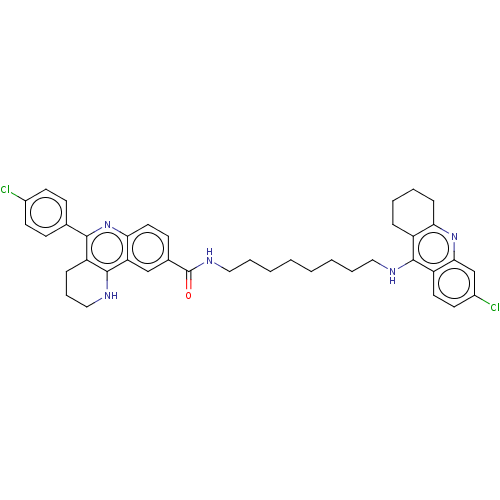 (CHEMBL3322231) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate by spectrophotometric analysis | Eur J Med Chem 84: 107-17 (2014) Article DOI: 10.1016/j.ejmech.2014.07.021 BindingDB Entry DOI: 10.7270/Q2571DNF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8987 (6-chloro-1,2,3,4-tetrahydroacridin-9-amine | 6-chl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate by spectrophotometric analysis | Eur J Med Chem 84: 107-17 (2014) Article DOI: 10.1016/j.ejmech.2014.07.021 BindingDB Entry DOI: 10.7270/Q2571DNF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50023607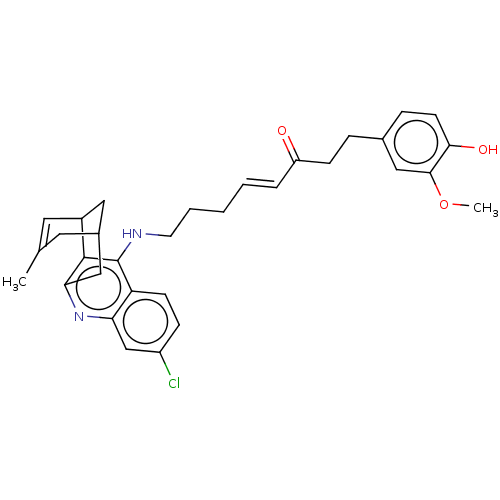 (CHEMBL3355579) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 20 mins before substrate addition by Ellman method | Bioorg Med Chem 22: 5298-307 (2014) Article DOI: 10.1016/j.bmc.2014.07.053 BindingDB Entry DOI: 10.7270/Q2M61MV9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50049861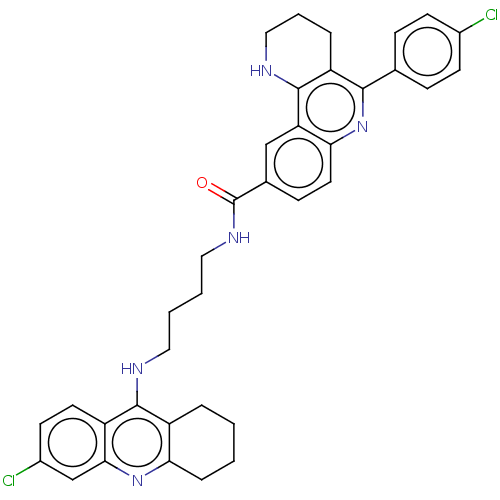 (CHEMBL3322233) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate by spectrophotometric analysis | Eur J Med Chem 84: 107-17 (2014) Article DOI: 10.1016/j.ejmech.2014.07.021 BindingDB Entry DOI: 10.7270/Q2571DNF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50049860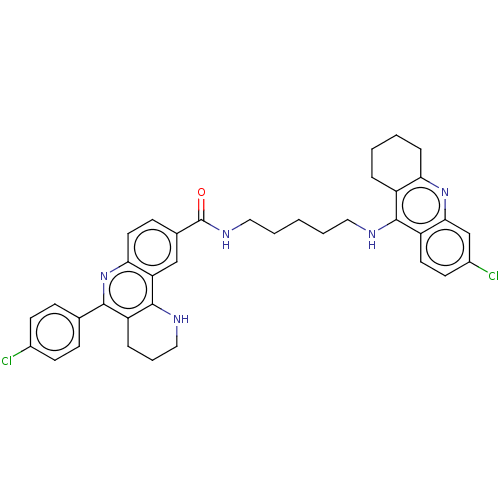 (CHEMBL3322234) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate by spectrophotometric analysis | Eur J Med Chem 84: 107-17 (2014) Article DOI: 10.1016/j.ejmech.2014.07.021 BindingDB Entry DOI: 10.7270/Q2571DNF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50023606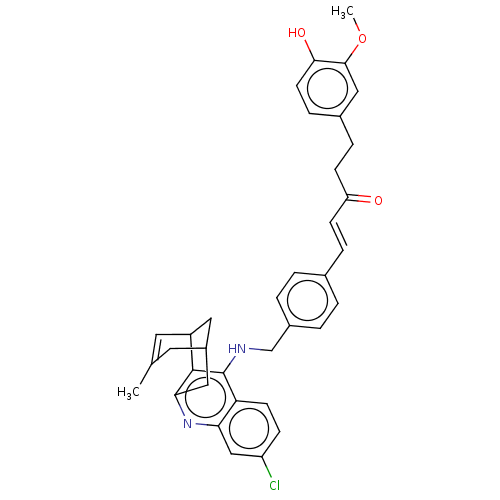 (CHEMBL3355580) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 20 mins before substrate addition by Ellman method | Bioorg Med Chem 22: 5298-307 (2014) Article DOI: 10.1016/j.bmc.2014.07.053 BindingDB Entry DOI: 10.7270/Q2M61MV9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM15579 (CHEMBL972 | DEPRENYL | L-Deprenyl | N-methyl-N-[(2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of human recombinant MAO-B preincubated for 30 mins followed by p-tyramine hydrochloride addition measured after 30 mins by Amplex red flu... | Bioorg Med Chem 24: 4835-4854 (2016) Article DOI: 10.1016/j.bmc.2016.06.045 BindingDB Entry DOI: 10.7270/Q2028W20 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM31895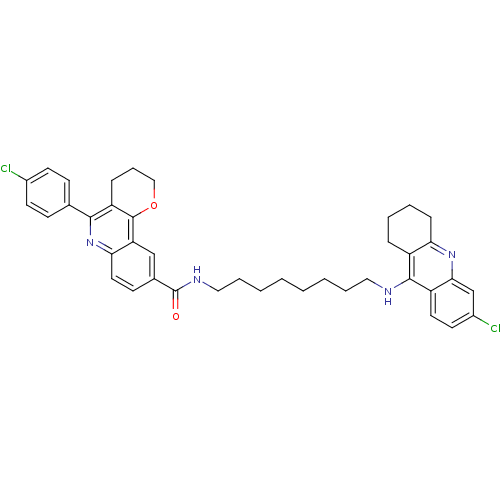 (Pyrano[3,2-c]quinoline-6-chlorotacrine hybrid, 20....) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate by spectrophotometric analysis | Eur J Med Chem 84: 107-17 (2014) Article DOI: 10.1016/j.ejmech.2014.07.021 BindingDB Entry DOI: 10.7270/Q2571DNF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM15581 (CHEMBL8706 | CLG | CLORGILINE | Clorgyline | N-[3-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of human recombinant MAO-A preincubated for 30 mins followed by p-tyramine hydrochloride addition measured after 30 mins by Amplex red flu... | Bioorg Med Chem 24: 4835-4854 (2016) Article DOI: 10.1016/j.bmc.2016.06.045 BindingDB Entry DOI: 10.7270/Q2028W20 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50023605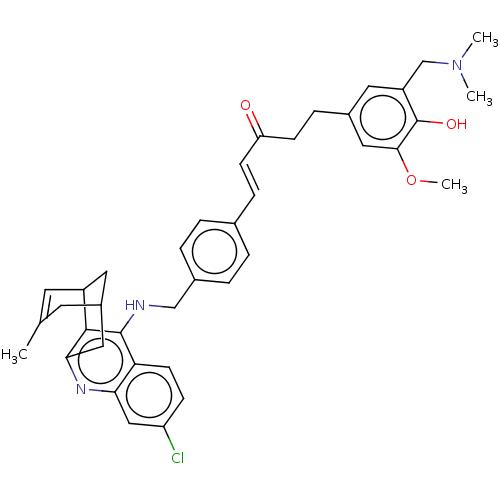 (CHEMBL3355581) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 20 mins before substrate addition by Ellman method | Bioorg Med Chem 22: 5298-307 (2014) Article DOI: 10.1016/j.bmc.2014.07.053 BindingDB Entry DOI: 10.7270/Q2M61MV9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50448131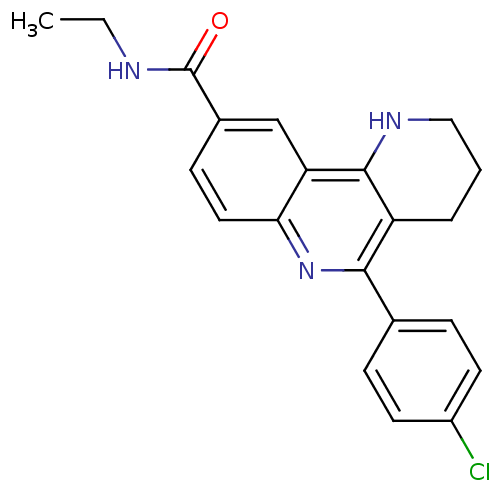 (CHEMBL3122167) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition measured after 5 ... | Eur J Med Chem 73: 141-52 (2014) Article DOI: 10.1016/j.ejmech.2013.12.008 BindingDB Entry DOI: 10.7270/Q2CF9RKN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50448131 (CHEMBL3122167) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate by spectrophotometric analysis | Eur J Med Chem 84: 107-17 (2014) Article DOI: 10.1016/j.ejmech.2014.07.021 BindingDB Entry DOI: 10.7270/Q2571DNF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM8987 (6-chloro-1,2,3,4-tetrahydroacridin-9-amine | 6-chl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 114 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of human recombinant BChE using butyrylthiocholine iodide as substrate by spectrophotometric analysis | Eur J Med Chem 84: 107-17 (2014) Article DOI: 10.1016/j.ejmech.2014.07.021 BindingDB Entry DOI: 10.7270/Q2571DNF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50049862 (CHEMBL3322232) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of human recombinant BChE using butyrylthiocholine iodide as substrate by spectrophotometric analysis | Eur J Med Chem 84: 107-17 (2014) Article DOI: 10.1016/j.ejmech.2014.07.021 BindingDB Entry DOI: 10.7270/Q2571DNF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50448132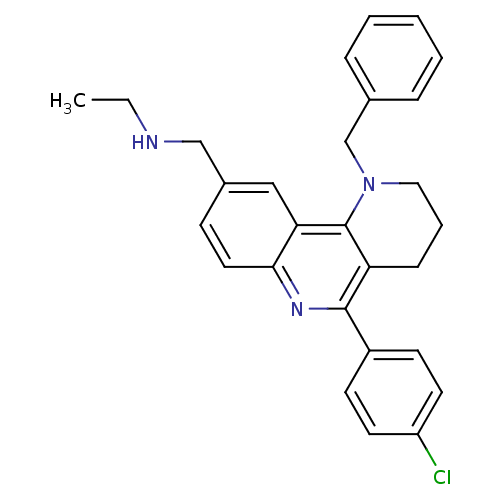 (CHEMBL3122150) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 147 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition measured after 5 ... | Eur J Med Chem 73: 141-52 (2014) Article DOI: 10.1016/j.ejmech.2013.12.008 BindingDB Entry DOI: 10.7270/Q2CF9RKN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50448135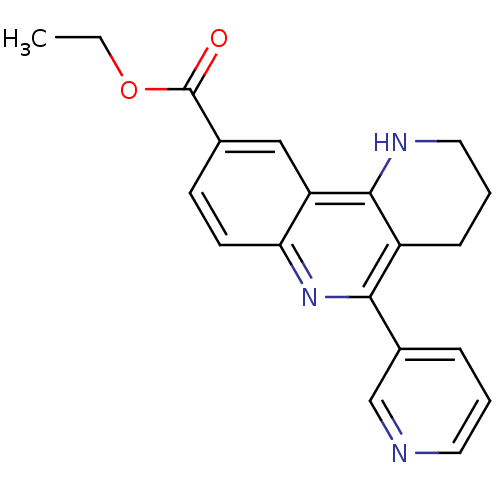 (CHEMBL3122166) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 148 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition measured after 5 ... | Eur J Med Chem 73: 141-52 (2014) Article DOI: 10.1016/j.ejmech.2013.12.008 BindingDB Entry DOI: 10.7270/Q2CF9RKN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM10592 (7-chloro-15-methyl-10-azatetracyclo[11.3.1.0^{2,11...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 175 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of human serum BChE using butyrylthiocholine iodide preincubated for 20 mins before substrate addition by Ellman method | Bioorg Med Chem 22: 5298-307 (2014) Article DOI: 10.1016/j.bmc.2014.07.053 BindingDB Entry DOI: 10.7270/Q2M61MV9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50023605 (CHEMBL3355581) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 181 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of human serum BChE using butyrylthiocholine iodide preincubated for 20 mins before substrate addition by Ellman method | Bioorg Med Chem 22: 5298-307 (2014) Article DOI: 10.1016/j.bmc.2014.07.053 BindingDB Entry DOI: 10.7270/Q2M61MV9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50049861 (CHEMBL3322233) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 205 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of human recombinant BChE using butyrylthiocholine iodide as substrate by spectrophotometric analysis | Eur J Med Chem 84: 107-17 (2014) Article DOI: 10.1016/j.ejmech.2014.07.021 BindingDB Entry DOI: 10.7270/Q2571DNF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM31895 (Pyrano[3,2-c]quinoline-6-chlorotacrine hybrid, 20....) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 223 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of human recombinant BChE using butyrylthiocholine iodide as substrate by spectrophotometric analysis | Eur J Med Chem 84: 107-17 (2014) Article DOI: 10.1016/j.ejmech.2014.07.021 BindingDB Entry DOI: 10.7270/Q2571DNF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50448138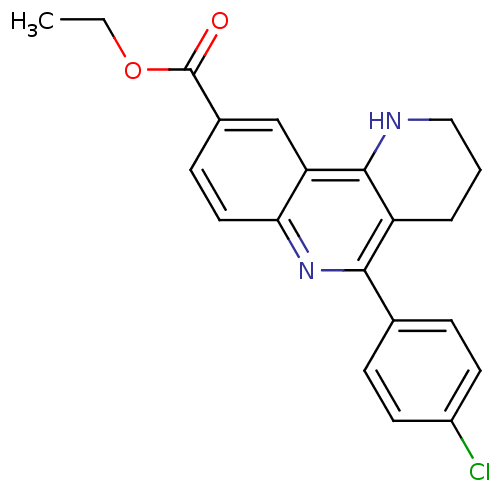 (CHEMBL3122147) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 281 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition measured after 5 ... | Eur J Med Chem 73: 141-52 (2014) Article DOI: 10.1016/j.ejmech.2013.12.008 BindingDB Entry DOI: 10.7270/Q2CF9RKN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50049859 (CHEMBL3322231) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 286 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of human recombinant BChE using butyrylthiocholine iodide as substrate by spectrophotometric analysis | Eur J Med Chem 84: 107-17 (2014) Article DOI: 10.1016/j.ejmech.2014.07.021 BindingDB Entry DOI: 10.7270/Q2571DNF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50049860 (CHEMBL3322234) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 337 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of human recombinant BChE using butyrylthiocholine iodide as substrate by spectrophotometric analysis | Eur J Med Chem 84: 107-17 (2014) Article DOI: 10.1016/j.ejmech.2014.07.021 BindingDB Entry DOI: 10.7270/Q2571DNF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50499520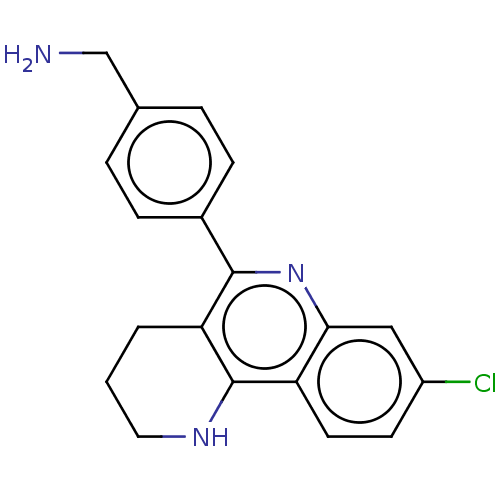 (CHEMBL3741559) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 440 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus acetylcholinesterase pre-incubated for 20 mins before acetylthiocholine iodide substrate addition by spectroph... | Eur J Med Chem 105: 120-37 (2015) Article DOI: 10.1016/j.ejmech.2015.10.007 BindingDB Entry DOI: 10.7270/Q2GF0XHB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50536264 (CHEMBL4592550) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of human recombinant MAO-A preincubated for 30 mins followed by p-tyramine hydrochloride addition measured after 30 mins by Amplex red flu... | Bioorg Med Chem 24: 4835-4854 (2016) Article DOI: 10.1016/j.bmc.2016.06.045 BindingDB Entry DOI: 10.7270/Q2028W20 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50448130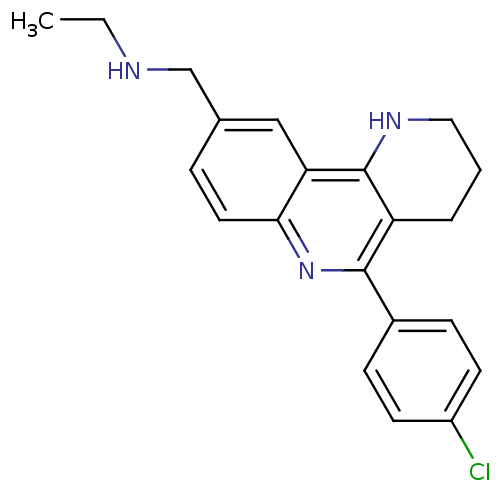 (CHEMBL3122168) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 532 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition measured after 5 ... | Eur J Med Chem 73: 141-52 (2014) Article DOI: 10.1016/j.ejmech.2013.12.008 BindingDB Entry DOI: 10.7270/Q2CF9RKN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50536264 (CHEMBL4592550) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of human recombinant MAO-B preincubated for 30 mins followed by p-tyramine hydrochloride addition measured after 30 mins by Amplex red flu... | Bioorg Med Chem 24: 4835-4854 (2016) Article DOI: 10.1016/j.bmc.2016.06.045 BindingDB Entry DOI: 10.7270/Q2028W20 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50023606 (CHEMBL3355580) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 742 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of human serum BChE using butyrylthiocholine iodide preincubated for 20 mins before substrate addition by Ellman method | Bioorg Med Chem 22: 5298-307 (2014) Article DOI: 10.1016/j.bmc.2014.07.053 BindingDB Entry DOI: 10.7270/Q2M61MV9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50499519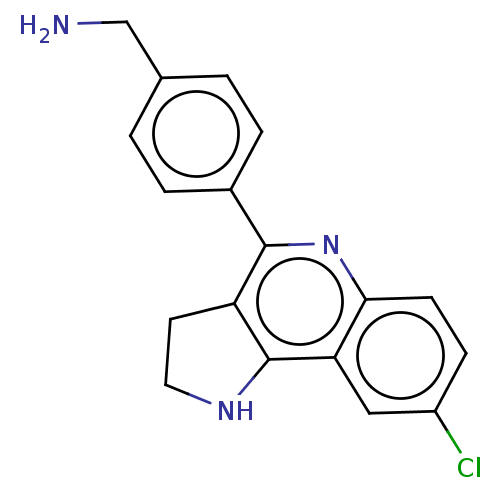 (CHEMBL3740980) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 870 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus acetylcholinesterase pre-incubated for 20 mins before acetylthiocholine iodide substrate addition by spectroph... | Eur J Med Chem 105: 120-37 (2015) Article DOI: 10.1016/j.ejmech.2015.10.007 BindingDB Entry DOI: 10.7270/Q2GF0XHB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50448131 (CHEMBL3122167) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 920 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of human recombinant BChE using butyrylthiocholine iodide as substrate by spectrophotometric analysis | Eur J Med Chem 84: 107-17 (2014) Article DOI: 10.1016/j.ejmech.2014.07.021 BindingDB Entry DOI: 10.7270/Q2571DNF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50499526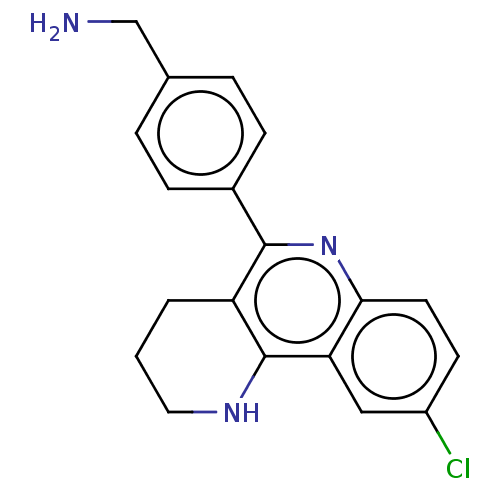 (CHEMBL3742126) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 970 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus acetylcholinesterase pre-incubated for 20 mins before acetylthiocholine iodide substrate addition by spectroph... | Eur J Med Chem 105: 120-37 (2015) Article DOI: 10.1016/j.ejmech.2015.10.007 BindingDB Entry DOI: 10.7270/Q2GF0XHB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50023607 (CHEMBL3355579) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 982 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of human serum BChE using butyrylthiocholine iodide preincubated for 20 mins before substrate addition by Ellman method | Bioorg Med Chem 22: 5298-307 (2014) Article DOI: 10.1016/j.bmc.2014.07.053 BindingDB Entry DOI: 10.7270/Q2M61MV9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50499516 (CHEMBL3739725) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.05E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus acetylcholinesterase pre-incubated for 20 mins before acetylthiocholine iodide substrate addition by spectroph... | Eur J Med Chem 105: 120-37 (2015) Article DOI: 10.1016/j.ejmech.2015.10.007 BindingDB Entry DOI: 10.7270/Q2GF0XHB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50499524 (CHEMBL3741060) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus acetylcholinesterase pre-incubated for 20 mins before acetylthiocholine iodide substrate addition by spectroph... | Eur J Med Chem 105: 120-37 (2015) Article DOI: 10.1016/j.ejmech.2015.10.007 BindingDB Entry DOI: 10.7270/Q2GF0XHB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50499517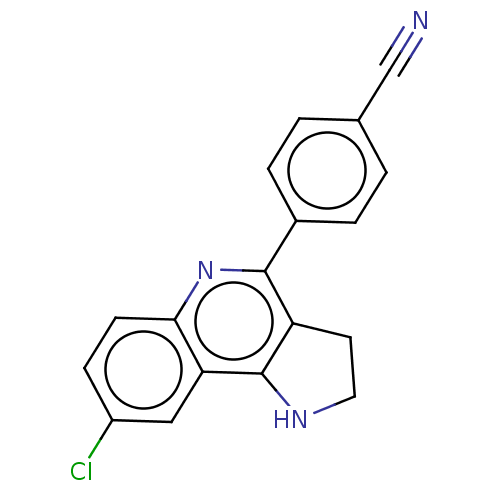 (CHEMBL3739464) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.19E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus acetylcholinesterase pre-incubated for 20 mins before acetylthiocholine iodide substrate addition by spectroph... | Eur J Med Chem 105: 120-37 (2015) Article DOI: 10.1016/j.ejmech.2015.10.007 BindingDB Entry DOI: 10.7270/Q2GF0XHB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50499525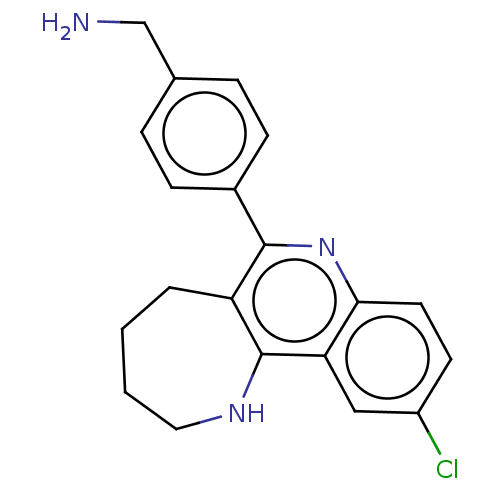 (CHEMBL3740568) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus acetylcholinesterase pre-incubated for 20 mins before acetylthiocholine iodide substrate addition by spectroph... | Eur J Med Chem 105: 120-37 (2015) Article DOI: 10.1016/j.ejmech.2015.10.007 BindingDB Entry DOI: 10.7270/Q2GF0XHB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50448140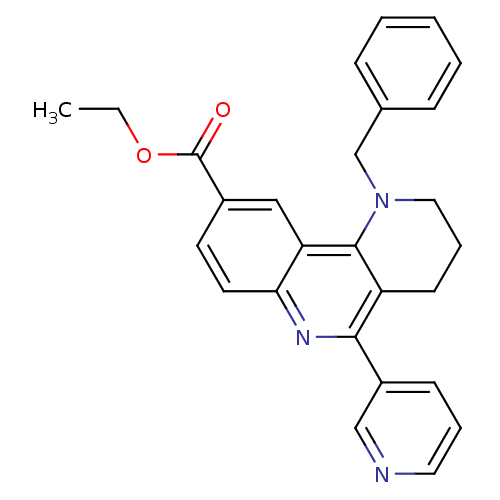 (CHEMBL3122145) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.97E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition measured after 5 ... | Eur J Med Chem 73: 141-52 (2014) Article DOI: 10.1016/j.ejmech.2013.12.008 BindingDB Entry DOI: 10.7270/Q2CF9RKN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM15579 (CHEMBL972 | DEPRENYL | L-Deprenyl | N-methyl-N-[(2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of human recombinant MAO-A preincubated for 30 mins followed by p-tyramine hydrochloride addition measured after 30 mins by Amplex red flu... | Bioorg Med Chem 24: 4835-4854 (2016) Article DOI: 10.1016/j.bmc.2016.06.045 BindingDB Entry DOI: 10.7270/Q2028W20 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50536257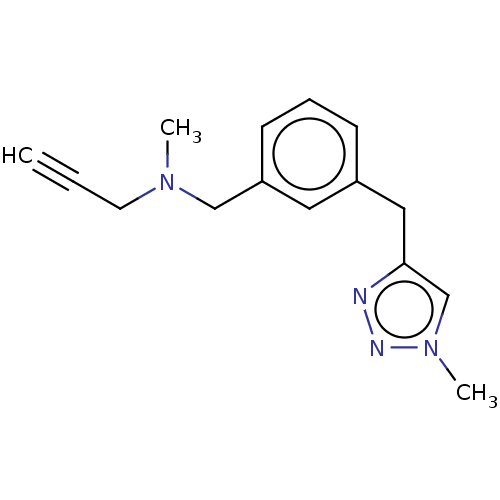 (CHEMBL4577856) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of human recombinant MAO-A preincubated for 30 mins followed by p-tyramine hydrochloride addition measured after 30 mins by Amplex red flu... | Bioorg Med Chem 24: 4835-4854 (2016) Article DOI: 10.1016/j.bmc.2016.06.045 BindingDB Entry DOI: 10.7270/Q2028W20 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50448134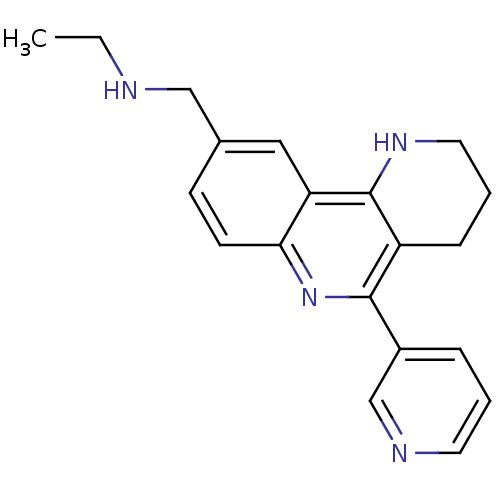 (CHEMBL3122169) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.15E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition measured after 5 ... | Eur J Med Chem 73: 141-52 (2014) Article DOI: 10.1016/j.ejmech.2013.12.008 BindingDB Entry DOI: 10.7270/Q2CF9RKN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50499518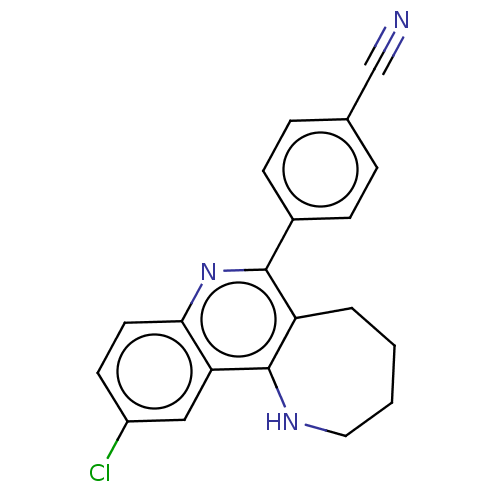 (CHEMBL3740006) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus acetylcholinesterase pre-incubated for 20 mins before acetylthiocholine iodide substrate addition by spectroph... | Eur J Med Chem 105: 120-37 (2015) Article DOI: 10.1016/j.ejmech.2015.10.007 BindingDB Entry DOI: 10.7270/Q2GF0XHB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50499521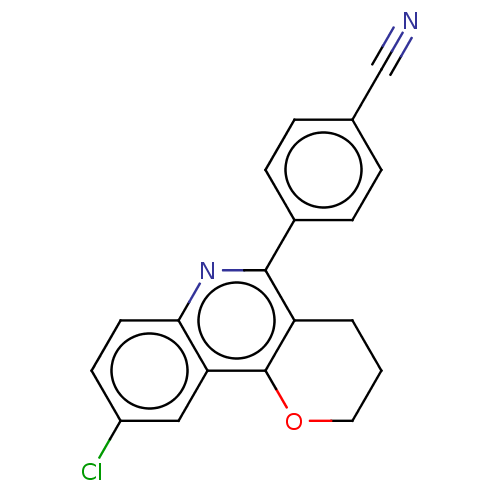 (CHEMBL3741468) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus acetylcholinesterase pre-incubated for 20 mins before acetylthiocholine iodide substrate addition by spectroph... | Eur J Med Chem 105: 120-37 (2015) Article DOI: 10.1016/j.ejmech.2015.10.007 BindingDB Entry DOI: 10.7270/Q2GF0XHB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50499515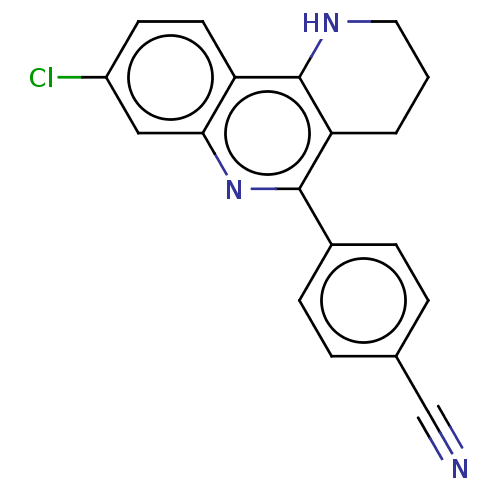 (CHEMBL3741946) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.27E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus acetylcholinesterase pre-incubated for 20 mins before acetylthiocholine iodide substrate addition by spectroph... | Eur J Med Chem 105: 120-37 (2015) Article DOI: 10.1016/j.ejmech.2015.10.007 BindingDB Entry DOI: 10.7270/Q2GF0XHB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50536260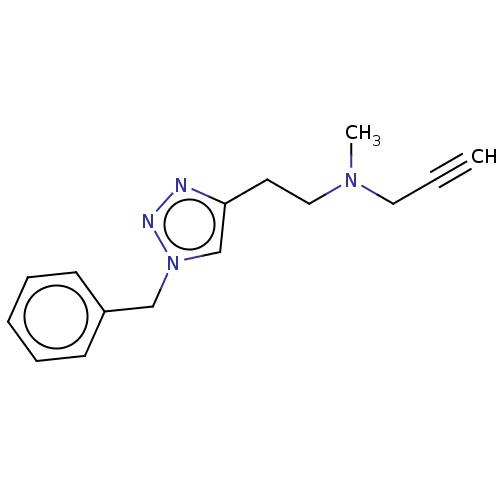 (CHEMBL4527885) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of human recombinant MAO-B preincubated for 30 mins followed by p-tyramine hydrochloride addition measured after 30 mins by Amplex red flu... | Bioorg Med Chem 24: 4835-4854 (2016) Article DOI: 10.1016/j.bmc.2016.06.045 BindingDB Entry DOI: 10.7270/Q2028W20 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50499528 (CHEMBL3741859) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.67E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus acetylcholinesterase pre-incubated for 20 mins before acetylthiocholine iodide substrate addition by spectroph... | Eur J Med Chem 105: 120-37 (2015) Article DOI: 10.1016/j.ejmech.2015.10.007 BindingDB Entry DOI: 10.7270/Q2GF0XHB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50536263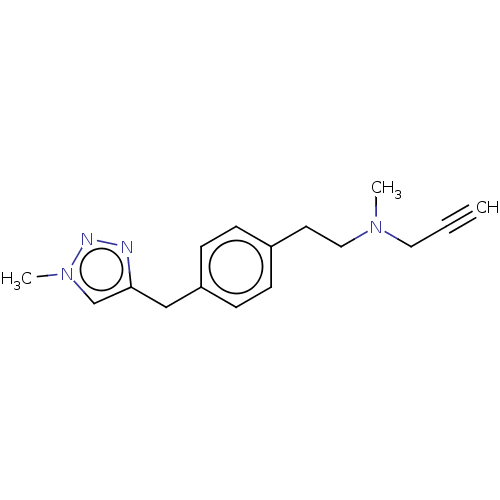 (CHEMBL4533245) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of human recombinant MAO-B preincubated for 30 mins followed by p-tyramine hydrochloride addition measured after 30 mins by Amplex red flu... | Bioorg Med Chem 24: 4835-4854 (2016) Article DOI: 10.1016/j.bmc.2016.06.045 BindingDB Entry DOI: 10.7270/Q2028W20 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50448133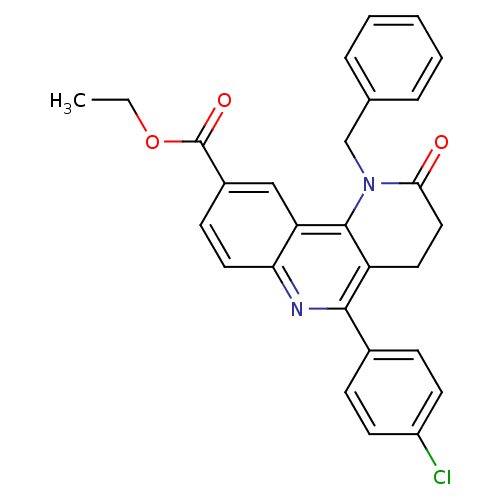 (CHEMBL3122170) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.21E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition measured after 5 ... | Eur J Med Chem 73: 141-52 (2014) Article DOI: 10.1016/j.ejmech.2013.12.008 BindingDB Entry DOI: 10.7270/Q2CF9RKN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50448136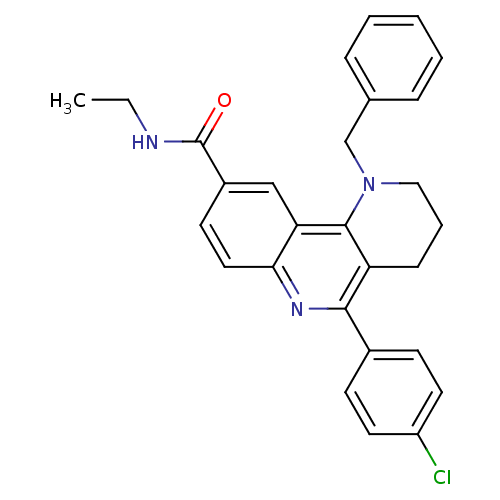 (CHEMBL3122149) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.48E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition measured after 5 ... | Eur J Med Chem 73: 141-52 (2014) Article DOI: 10.1016/j.ejmech.2013.12.008 BindingDB Entry DOI: 10.7270/Q2CF9RKN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 90 total ) | Next | Last >> |