| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Cytochrome P450 1A2 |
|---|
| Ligand | BDBM50079578 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_1471686 (CHEMBL3421054) |
|---|
| IC50 | >30000±n/a nM |
|---|
| Citation |  Hudkins, RL; Becknell, NC; Lyons, JA; Aimone, LD; Olsen, M; Haltiwanger, RC; Mathiasen, JR; Raddatz, R; Gruner, JA 3,4-Diaza-bicyclo[4.1.0]hept-4-en-2-one phenoxypropylamine analogs of irdabisant (CEP-26401) as potent histamine-3 receptor inverse agonists with robust wake-promoting activity. Eur J Med Chem95:349-56 (2015) [PubMed] Article Hudkins, RL; Becknell, NC; Lyons, JA; Aimone, LD; Olsen, M; Haltiwanger, RC; Mathiasen, JR; Raddatz, R; Gruner, JA 3,4-Diaza-bicyclo[4.1.0]hept-4-en-2-one phenoxypropylamine analogs of irdabisant (CEP-26401) as potent histamine-3 receptor inverse agonists with robust wake-promoting activity. Eur J Med Chem95:349-56 (2015) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Cytochrome P450 1A2 |
|---|
| Name: | Cytochrome P450 1A2 |
|---|
| Synonyms: | CP1A2_HUMAN | CYP1A2 | CYPIA2 | Cholesterol 25-hydroxylase | Cytochrome P(3)450 | Cytochrome P450 1A | Cytochrome P450 1A2 (CYP1A2) | Cytochrome P450 4 | Cytochrome P450-P3 |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 58423.38 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P05177 |
|---|
| Residue: | 516 |
|---|
| Sequence: | MALSQSVPFSATELLLASAIFCLVFWVLKGLRPRVPKGLKSPPEPWGWPLLGHVLTLGKN
PHLALSRMSQRYGDVLQIRIGSTPVLVLSRLDTIRQALVRQGDDFKGRPDLYTSTLITDG
QSLTFSTDSGPVWAARRRLAQNALNTFSIASDPASSSSCYLEEHVSKEAKALISRLQELM
AGPGHFDPYNQVVVSVANVIGAMCFGQHFPESSDEMLSLVKNTHEFVETASSGNPLDFFP
ILRYLPNPALQRFKAFNQRFLWFLQKTVQEHYQDFDKNSVRDITGALFKHSKKGPRASGN
LIPQEKIVNLVNDIFGAGFDTVTTAISWSLMYLVTKPEIQRKIQKELDTVIGRERRPRLS
DRPQLPYLEAFILETFRHSSFLPFTIPHSTTRDTTLNGFYIPKKCCVFVNQWQVNHDPEL
WEDPSEFRPERFLTADGTAINKPLSEKMMLFGMGKRRCIGEVLAKWEIFLFLAILLQQLE
FSVPPGVKVDLTPIYGLTMKHARCEHVQARLRFSIN
|
|
|
|---|
| BDBM50079578 |
|---|
| n/a |
|---|
| Name | BDBM50079578 |
|---|
| Synonyms: | CHEMBL3417504 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C19H25N3O2 |
|---|
| Mol. Mass. | 327.4207 |
|---|
| SMILES | C[C@@H]1CCCN1CCCOc1ccc(cc1)C1=NNC(=O)C2CC12 |r,t:18| |
|---|
| Structure | 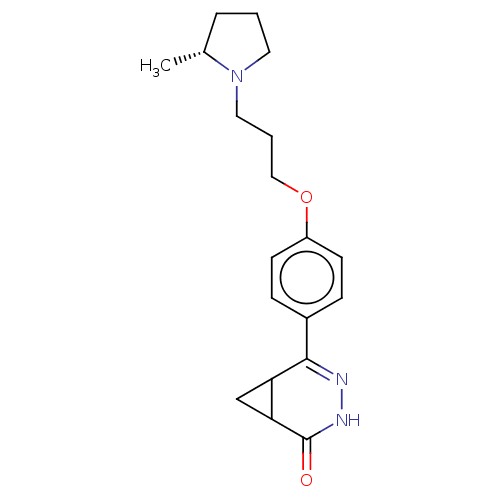
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Hudkins, RL; Becknell, NC; Lyons, JA; Aimone, LD; Olsen, M; Haltiwanger, RC; Mathiasen, JR; Raddatz, R; Gruner, JA 3,4-Diaza-bicyclo[4.1.0]hept-4-en-2-one phenoxypropylamine analogs of irdabisant (CEP-26401) as potent histamine-3 receptor inverse agonists with robust wake-promoting activity. Eur J Med Chem95:349-56 (2015) [PubMed] Article
Hudkins, RL; Becknell, NC; Lyons, JA; Aimone, LD; Olsen, M; Haltiwanger, RC; Mathiasen, JR; Raddatz, R; Gruner, JA 3,4-Diaza-bicyclo[4.1.0]hept-4-en-2-one phenoxypropylamine analogs of irdabisant (CEP-26401) as potent histamine-3 receptor inverse agonists with robust wake-promoting activity. Eur J Med Chem95:349-56 (2015) [PubMed] Article