

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Reaction Details | |||
|---|---|---|---|
 | Report a problem with these data | ||
| Target | Tyrosine-protein phosphatase non-receptor type 1 | ||
| Ligand | BDBM50259862 | ||
| Substrate/Competitor | n/a | ||
| Meas. Tech. | ChEMBL_1501267 (CHEMBL3587976) | ||
| Ki | 4600±n/a nM | ||
| Citation |  Nguyen, PH; Ji, DJ; Han, YR; Choi, JS; Rhyu, DY; Min, BS; Woo, MH Selaginellin and biflavonoids as protein tyrosine phosphatase 1B inhibitors from Selaginella tamariscina and their glucose uptake stimulatory effects. Bioorg Med Chem23:3730-7 (2015) [PubMed] Article Nguyen, PH; Ji, DJ; Han, YR; Choi, JS; Rhyu, DY; Min, BS; Woo, MH Selaginellin and biflavonoids as protein tyrosine phosphatase 1B inhibitors from Selaginella tamariscina and their glucose uptake stimulatory effects. Bioorg Med Chem23:3730-7 (2015) [PubMed] Article | ||
| More Info.: | Get all data from this article, Assay Method | ||
| Tyrosine-protein phosphatase non-receptor type 1 | |||
| Name: | Tyrosine-protein phosphatase non-receptor type 1 | ||
| Synonyms: | PTN1_HUMAN | PTP1B | PTPN1 | Protein tyrosine phosphatase 1B (PTP1B) | Protein tyrosine phosphatase-1B (PTP1B) | Protein-tyrosine phosphatase 1B | Protein-tyrosine phosphatase 1B (PTP1B) | Tyrosine-protein phosphatase non-receptor type 1 | Tyrosine-protein phosphatase non-receptor type 1 (PTP1B) | ||
| Type: | Protein phosphatase | ||
| Mol. Mass.: | 49963.76 | ||
| Organism: | Homo sapiens (Human) | ||
| Description: | Human recombinant GST-fusion PTP1B (1-435). | ||
| Residue: | 435 | ||
| Sequence: |
| ||
| BDBM50259862 | |||
| n/a | |||
| Name | BDBM50259862 | ||
| Synonyms: | 13,II8-biapigenin | 3,8''-biapigenin | CHEMBL515252 | I3,II8-biapigenin | cid_10414856 | ||
| Type | Small organic molecule | ||
| Emp. Form. | C30H18O10 | ||
| Mol. Mass. | 538.4579 | ||
| SMILES | Oc1ccc(cc1)-c1cc(=O)c2c(O)cc(O)c(-c3c(oc4cc(O)cc(O)c4c3=O)-c3ccc(O)cc3)c2o1 |(4.11,-9.88,;3.39,-11.25,;4.21,-12.55,;3.49,-13.91,;1.94,-13.97,;1.13,-12.67,;1.84,-11.31,;1.22,-15.32,;2.03,-16.61,;1.32,-17.95,;2.13,-19.25,;-.19,-18,;-.92,-19.36,;-.11,-20.67,;-2.45,-19.41,;-3.25,-18.11,;-4.31,-19.19,;-2.52,-16.76,;-3.32,-15.46,;-3.32,-13.91,;-4.67,-13.14,;-6,-13.93,;-7.34,-13.16,;-8.67,-13.94,;-10.01,-13.17,;-8.67,-15.48,;-7.34,-16.25,;-7.34,-17.8,;-6,-15.48,;-4.66,-16.24,;-4.56,-17.65,;-2.16,-12.91,;-.7,-13.43,;.47,-12.42,;.18,-10.91,;1.35,-9.9,;-1.28,-10.4,;-2.45,-11.41,;-1,-16.72,;-.3,-15.38,)| | ||
| Structure | 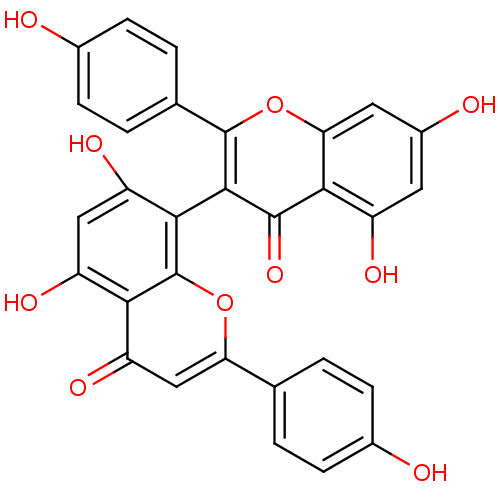
| ||