| Reaction Details |
|---|
 | Report a problem with these data |
| Target | 5-hydroxytryptamine receptor 2C |
|---|
| Ligand | BDBM50132341 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_1527393 (CHEMBL3636768) |
|---|
| Ki | <300±n/a nM |
|---|
| Citation |  Ponnala, S; Kapadia, N; Madapa, S; Alberts, IL; Harding, WW Synthesis and evaluation of aporphine analogs containing C1 allyl isosteres at the h5-HT(2A) receptor. Bioorg Med Chem Lett25:5102-6 (2015) [PubMed] Article Ponnala, S; Kapadia, N; Madapa, S; Alberts, IL; Harding, WW Synthesis and evaluation of aporphine analogs containing C1 allyl isosteres at the h5-HT(2A) receptor. Bioorg Med Chem Lett25:5102-6 (2015) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| 5-hydroxytryptamine receptor 2C |
|---|
| Name: | 5-hydroxytryptamine receptor 2C |
|---|
| Synonyms: | 5-HT-1C | 5-HT-2C | 5-HT1C | 5-HT2C | 5-HT2C-INI | 5-HT2c VGI | 5-HTR2C | 5-hydroxytryptamine receptor 1C | 5-hydroxytryptamine receptor 2C (5-HT-2C) | 5-hydroxytryptamine receptor 2C (5HT-2C) | 5HT-1C | 5HT2C_HUMAN | HTR1C | HTR2C | Serotonin (5-HT3) receptor | Serotonin 2c (5-HT2c) receptor | Serotonin Receptor 2C |
|---|
| Type: | G Protein-Coupled Receptor (GPCR) |
|---|
| Mol. Mass.: | 51836.79 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P28335 |
|---|
| Residue: | 458 |
|---|
| Sequence: | MVNLRNAVHSFLVHLIGLLVWQSDISVSPVAAIVTDIFNTSDGGRFKFPDGVQNWPALSI
VIIIIMTIGGNILVIMAVSMEKKLHNATNYFLMSLAIADMLVGLLVMPLSLLAILYDYVW
PLPRYLCPVWISLDVLFSTASIMHLCAISLDRYVAIRNPIEHSRFNSRTKAIMKIAIVWA
ISIGVSVPIPVIGLRDEEKVFVNNTTCVLNDPNFVLIGSFVAFFIPLTIMVITYCLTIYV
LRRQALMLLHGHTEEPPGLSLDFLKCCKRNTAEEENSANPNQDQNARRRKKKERRPRGTM
QAINNERKASKVLGIVFFVFLIMWCPFFITNILSVLCEKSCNQKLMEKLLNVFVWIGYVC
SGINPLVYTLFNKIYRRAFSNYLRCNYKVEKKPPVRQIPRVAATALSGRELNVNIYRHTN
EPVIEKASDNEPGIEMQVENLELPVNPSSVVSERISSV
|
|
|
|---|
| BDBM50132341 |
|---|
| n/a |
|---|
| Name | BDBM50132341 |
|---|
| Synonyms: | CHEMBL3633757 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C26H24BrNO4 |
|---|
| Mol. Mass. | 494.377 |
|---|
| SMILES | COc1cc2CCN(C)C3Cc4cc5OCOc5cc4-c(c1OCc1ccc(Br)cc1)c23 |
|---|
| Structure | 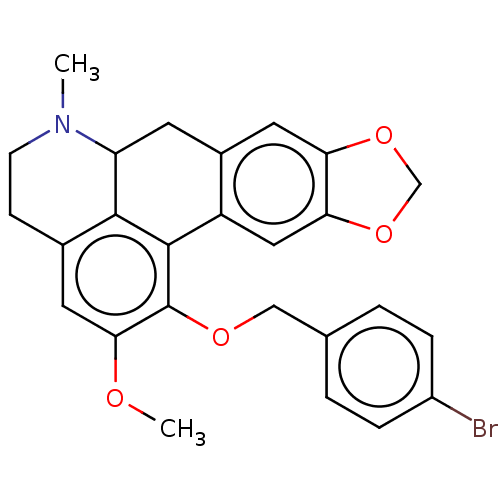
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Ponnala, S; Kapadia, N; Madapa, S; Alberts, IL; Harding, WW Synthesis and evaluation of aporphine analogs containing C1 allyl isosteres at the h5-HT(2A) receptor. Bioorg Med Chem Lett25:5102-6 (2015) [PubMed] Article
Ponnala, S; Kapadia, N; Madapa, S; Alberts, IL; Harding, WW Synthesis and evaluation of aporphine analogs containing C1 allyl isosteres at the h5-HT(2A) receptor. Bioorg Med Chem Lett25:5102-6 (2015) [PubMed] Article