 Found 981 hits with Last Name = 'harding' and Initial = 'ww'
Found 981 hits with Last Name = 'harding' and Initial = 'ww' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM82247

(8-Chloro-3-methyl-5-phenyl-2,3,4,5-tetrahydro-1H-b...)Show InChI InChI=1S/C17H18ClNO/c1-19-8-7-13-9-16(18)17(20)10-14(13)15(11-19)12-5-3-2-4-6-12/h2-6,9-10,15,20H,7-8,11H2,1H3/t15-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
City University of New York
Curated by ChEMBL
| Assay Description
Antagonist activity at dopamine D1 receptor (unknown origin) |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.127305
BindingDB Entry DOI: 10.7270/Q2377D85 |
More data for this
Ligand-Target Pair | |
D(1B) dopamine receptor
(Homo sapiens (Human)) | BDBM82247

(8-Chloro-3-methyl-5-phenyl-2,3,4,5-tetrahydro-1H-b...)Show InChI InChI=1S/C17H18ClNO/c1-19-8-7-13-9-16(18)17(20)10-14(13)15(11-19)12-5-3-2-4-6-12/h2-6,9-10,15,20H,7-8,11H2,1H3/t15-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
City University of New York
Curated by ChEMBL
| Assay Description
Antagonist activity at dopamine D5 receptor (unknown origin) |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.127305
BindingDB Entry DOI: 10.7270/Q2377D85 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1B
(Homo sapiens (Human)) | BDBM50249134
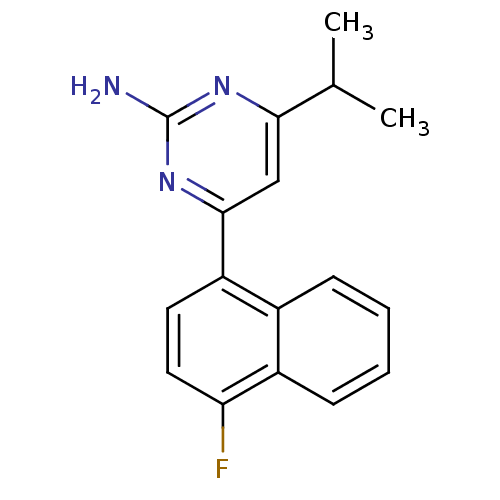
(4-(4-fluoronaphthalen-1-yl)-6-isopropylpyrimidin-2...)Show InChI InChI=1S/C17H16FN3/c1-10(2)15-9-16(21-17(19)20-15)13-7-8-14(18)12-6-4-3-5-11(12)13/h3-10H,1-2H3,(H2,19,20,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
City University of New York
Curated by ChEMBL
| Assay Description
Binding affinity to 5HT1B receptor (unknown origin) |
Bioorg Med Chem Lett 25: 3451-4 (2015)
Article DOI: 10.1016/j.bmcl.2015.07.012
BindingDB Entry DOI: 10.7270/Q2MW2JXN |
More data for this
Ligand-Target Pair | |
D(1B) dopamine receptor
(Homo sapiens (Human)) | BDBM50004822
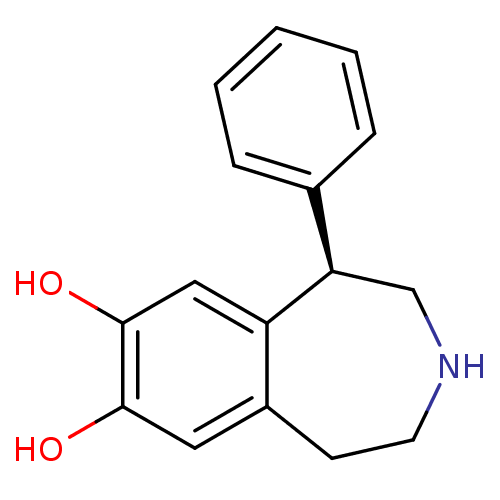
((R)-1-phenyl-2,3,4,5-tetrahydro-1H-benzo[d]azepine...)Show InChI InChI=1S/C16H17NO2/c18-15-8-12-6-7-17-10-14(13(12)9-16(15)19)11-4-2-1-3-5-11/h1-5,8-9,14,17-19H,6-7,10H2/t14-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
City University of New York
Curated by ChEMBL
| Assay Description
Agonist activity at dopamine D5 receptor (unknown origin) |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.127305
BindingDB Entry DOI: 10.7270/Q2377D85 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM21393

(7-(dipropylamino)-5,6,7,8-tetrahydronaphthalen-1-o...)Show InChI InChI=1S/C16H25NO/c1-3-10-17(11-4-2)14-9-8-13-6-5-7-16(18)15(13)12-14/h5-7,14,18H,3-4,8-12H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
City University of New York
Curated by ChEMBL
| Assay Description
Displacement of [3H]Way100635 from human recombinant 5-HT1A receptor expressed in CHO cell membranes by radioligand binding assay |
J Nat Prod 78: 722-9 (2015)
Article DOI: 10.1021/np500893h
BindingDB Entry DOI: 10.7270/Q23B61WB |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50487259
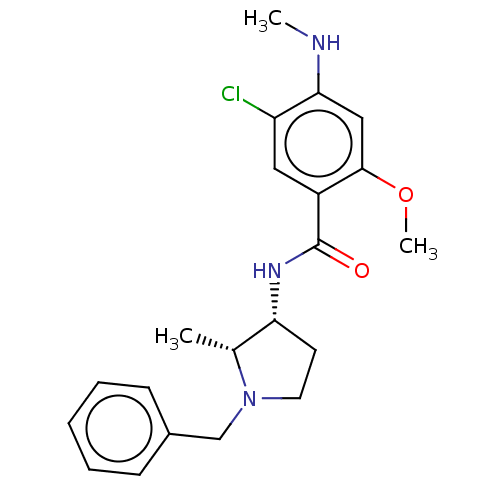
(CHEBI:64219 | [3H]NEMONAPRIDE)Show SMILES CNc1cc(OC)c(cc1Cl)C(=O)N[C@@H]1CCN(Cc2ccccc2)[C@@H]1C |r| Show InChI InChI=1S/C21H26ClN3O2/c1-14-18(9-10-25(14)13-15-7-5-4-6-8-15)24-21(26)16-11-17(22)19(23-2)12-20(16)27-3/h4-8,11-12,14,18,23H,9-10,13H2,1-3H3,(H,24,26)/t14-,18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 0.75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-methylspiperone from dopamine D4 receptor (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128047
BindingDB Entry DOI: 10.7270/Q2CJ8J8S |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50001775

((ritanserin)6-(2-{4-[Bis-(4-fluoro-phenyl)-methyle...)Show SMILES [#6]-c1nc2sccn2c(=O)c1-[#6]-[#6]-[#7]-1-[#6]-[#6]\[#6](-[#6]-[#6]-1)=[#6](\c1ccc(F)cc1)-c1ccc(F)cc1 Show InChI InChI=1S/C27H25F2N3OS/c1-18-24(26(33)32-16-17-34-27(32)30-18)12-15-31-13-10-21(11-14-31)25(19-2-6-22(28)7-3-19)20-4-8-23(29)9-5-20/h2-9,16-17H,10-15H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jnatprod.2c00365
BindingDB Entry DOI: 10.7270/Q2GX4GN3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM21393

(7-(dipropylamino)-5,6,7,8-tetrahydronaphthalen-1-o...)Show InChI InChI=1S/C16H25NO/c1-3-10-17(11-4-2)14-9-8-13-6-5-7-16(18)15(13)12-14/h5-7,14,18H,3-4,8-12H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
City University of New York
Curated by ChEMBL
| Assay Description
Displacement of [3H]-WAY100635 from human 5-HT1A receptor expresssed in stable CHO cell membrane incubated for 90 mins by microbeta counting method |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2020.115578
BindingDB Entry DOI: 10.7270/Q22B92KJ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM21393

(7-(dipropylamino)-5,6,7,8-tetrahydronaphthalen-1-o...)Show InChI InChI=1S/C16H25NO/c1-3-10-17(11-4-2)14-9-8-13-6-5-7-16(18)15(13)12-14/h5-7,14,18H,3-4,8-12H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
City University of New York
Curated by ChEMBL
| Assay Description
Binding affinity to human 5HT1A receptor |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.127053
BindingDB Entry DOI: 10.7270/Q2H135KF |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50487259

(CHEBI:64219 | [3H]NEMONAPRIDE)Show SMILES CNc1cc(OC)c(cc1Cl)C(=O)N[C@@H]1CCN(Cc2ccccc2)[C@@H]1C |r| Show InChI InChI=1S/C21H26ClN3O2/c1-14-18(9-10-25(14)13-15-7-5-4-6-8-15)24-21(26)16-11-17(22)19(23-2)12-20(16)27-3/h4-8,11-12,14,18,23H,9-10,13H2,1-3H3,(H,24,26)/t14-,18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 0.860 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
City University of New York
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-Methylspiperone from human dopamine D4 receptor expressed in stable HEK cells incubated for 90 mins by microbeta counting meth... |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2020.115578
BindingDB Entry DOI: 10.7270/Q22B92KJ |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens) | BDBM50487259

(CHEBI:64219 | [3H]NEMONAPRIDE)Show SMILES CNc1cc(OC)c(cc1Cl)C(=O)N[C@@H]1CCN(Cc2ccccc2)[C@@H]1C |r| Show InChI InChI=1S/C21H26ClN3O2/c1-14-18(9-10-25(14)13-15-7-5-4-6-8-15)24-21(26)16-11-17(22)19(23-2)12-20(16)27-3/h4-8,11-12,14,18,23H,9-10,13H2,1-3H3,(H,24,26)/t14-,18-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 0.860 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-methylspiperone from dopamine D3 receptor (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128047
BindingDB Entry DOI: 10.7270/Q2CJ8J8S |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM50004822

((R)-1-phenyl-2,3,4,5-tetrahydro-1H-benzo[d]azepine...)Show InChI InChI=1S/C16H17NO2/c18-15-8-12-6-7-17-10-14(13(12)9-16(15)19)11-4-2-1-3-5-11/h1-5,8-9,14,17-19H,6-7,10H2/t14-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
City University of New York
Curated by ChEMBL
| Assay Description
Agonist activity at dopamine D1 receptor (unknown origin) |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.127305
BindingDB Entry DOI: 10.7270/Q2377D85 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50087033

((1R,5aR)-1-methoxy-1,6-dimethyl-1,5,5a,6,7,8-hexah...)Show SMILES CO[C@]1(C)c2cccc3C[C@H]4N(C)CCc5ccc1c(-c23)c45 Show InChI InChI=1S/C20H21NO/c1-20(22-3)14-6-4-5-13-11-16-18-12(9-10-21(16)2)7-8-15(20)19(18)17(13)14/h4-8,16H,9-11H2,1-3H3/t16-,20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
City University of New York
Curated by ChEMBL
| Assay Description
Displacement of [3H]-LSD from human 5-HT7 receptor expresssed in stable HEK cell membrane incubated for 90 mins by microbeta counting method |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2020.115578
BindingDB Entry DOI: 10.7270/Q22B92KJ |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Homo sapiens (Human)) | BDBM21398

(4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...)Show SMILES OC1(CCN(CCCC(=O)c2ccc(F)cc2)CC1)c1ccc(Cl)cc1 Show InChI InChI=1S/C21H23ClFNO2/c22-18-7-5-17(6-8-18)21(26)11-14-24(15-12-21)13-1-2-20(25)16-3-9-19(23)10-4-16/h3-10,26H,1-2,11-15H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
City University of New York
Curated by ChEMBL
| Assay Description
Displacement of [3H](+)-pentazocine from human sigma1 receptor by PDSP assay |
Bioorg Med Chem 24: 2060-71 (2016)
Article DOI: 10.1016/j.bmc.2016.03.037
BindingDB Entry DOI: 10.7270/Q2SX6G3G |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
D(3) dopamine receptor
(Homo sapiens) | BDBM50570808
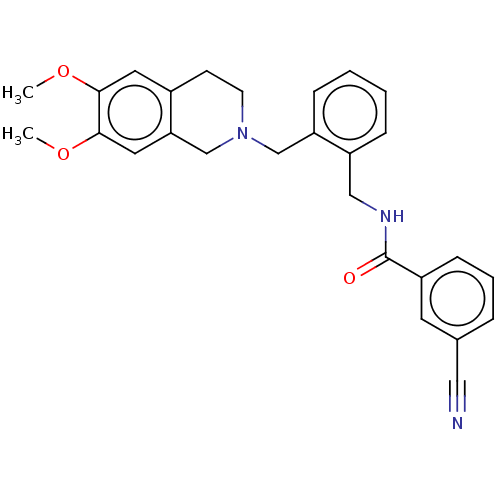
(CHEMBL4846574)Show SMILES COc1cc2CCN(Cc3ccccc3CNC(=O)c3cccc(c3)C#N)Cc2cc1OC | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-methylspiperone from dopamine D3 receptor (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128047
BindingDB Entry DOI: 10.7270/Q2CJ8J8S |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50487259

(CHEBI:64219 | [3H]NEMONAPRIDE)Show SMILES CNc1cc(OC)c(cc1Cl)C(=O)N[C@@H]1CCN(Cc2ccccc2)[C@@H]1C |r| Show InChI InChI=1S/C21H26ClN3O2/c1-14-18(9-10-25(14)13-15-7-5-4-6-8-15)24-21(26)16-11-17(22)19(23-2)12-20(16)27-3/h4-8,11-12,14,18,23H,9-10,13H2,1-3H3,(H,24,26)/t14-,18-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
City University of New York
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-Methylspiperone from dopamine D3 receptor (unknown origin) incubated for 90 mins by microbeta counting method |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2020.115578
BindingDB Entry DOI: 10.7270/Q22B92KJ |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM50210757
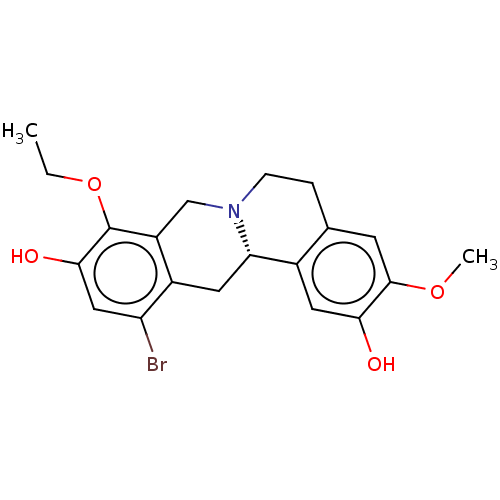
(CHEMBL3921278)Show SMILES [H][C@@]12Cc3c(Br)cc(O)c(OCC)c3CN1CCc1cc(OC)c(O)cc21 |r| Show InChI InChI=1S/C20H22BrNO4/c1-3-26-20-14-10-22-5-4-11-6-19(25-2)17(23)8-12(11)16(22)7-13(14)15(21)9-18(20)24/h6,8-9,16,23-24H,3-5,7,10H2,1-2H3/t16-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
City University of New York
Curated by ChEMBL
| Assay Description
Displacement of [3H]SCH23390 from human D1 receptor expressed in HEKT cell membranes after 90 mins by microbeta scintillation counting method |
Eur J Med Chem 125: 255-268 (2017)
Article DOI: 10.1016/j.ejmech.2016.09.036
BindingDB Entry DOI: 10.7270/Q2KW5J1T |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(Homo sapiens (Human)) | BDBM50178978

(CHEMBL3815112)Show SMILES COc1ccc(CCN(CCCc2ccccc2F)CCc2ccc3OCOc3c2)cc1OC Show InChI InChI=1S/C28H32FNO4/c1-31-25-11-9-21(18-27(25)32-2)13-16-30(15-5-7-23-6-3-4-8-24(23)29)17-14-22-10-12-26-28(19-22)34-20-33-26/h3-4,6,8-12,18-19H,5,7,13-17,20H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
City University Of New York
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-HT2B receptor measured after 90 mins by microbeta scintillation counting method |
Bioorg Med Chem Lett 26: 3216-3219 (2016)
Article DOI: 10.1016/j.bmcl.2016.05.079
BindingDB Entry DOI: 10.7270/Q29S1SXB |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50001775

((ritanserin)6-(2-{4-[Bis-(4-fluoro-phenyl)-methyle...)Show SMILES [#6]-c1nc2sccn2c(=O)c1-[#6]-[#6]-[#7]-1-[#6]-[#6]\[#6](-[#6]-[#6]-1)=[#6](\c1ccc(F)cc1)-c1ccc(F)cc1 Show InChI InChI=1S/C27H25F2N3OS/c1-18-24(26(33)32-16-17-34-27(32)30-18)12-15-31-13-10-21(11-14-31)25(19-2-6-22(28)7-3-19)20-4-8-23(29)9-5-20/h2-9,16-17H,10-15H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
City University Of New York
Curated by ChEMBL
| Assay Description
Displacement of [3H]mesulergine from human 5-HT2C receptor measured after 90 mins by microbeta scintillation counting method |
Bioorg Med Chem Lett 26: 3216-3219 (2016)
Article DOI: 10.1016/j.bmcl.2016.05.079
BindingDB Entry DOI: 10.7270/Q29S1SXB |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50170672
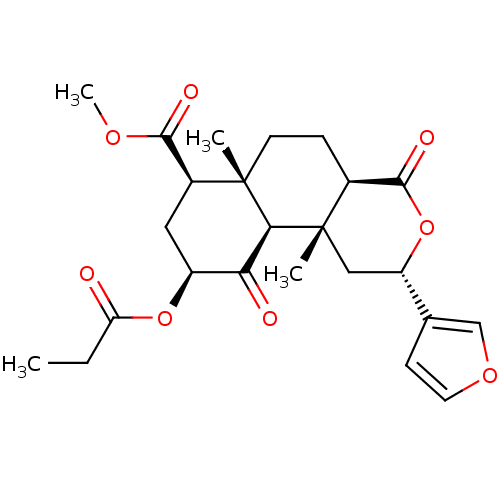
((2S,4aR,6aR,7R,9S,10aS,10bR)-methyl 2-(furan-3-yl)...)Show SMILES CCC(=O)O[C@H]1C[C@@H](C(=O)OC)[C@]2(C)CC[C@H]3C(=O)O[C@@H](C[C@]3(C)[C@H]2C1=O)c1ccoc1 |r| Show InChI InChI=1S/C24H30O8/c1-5-18(25)31-16-10-15(21(27)29-4)23(2)8-6-14-22(28)32-17(13-7-9-30-12-13)11-24(14,3)20(23)19(16)26/h7,9,12,14-17,20H,5-6,8,10-11H2,1-4H3/t14-,15-,16-,17-,20-,23-,24-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Iowa
Curated by ChEMBL
| Assay Description
Displacement of [125]OXY from kappa opioid receptor |
J Nat Prod 69: 914-8 (2006)
Article DOI: 10.1021/np060094b
BindingDB Entry DOI: 10.7270/Q28W3D22 |
More data for this
Ligand-Target Pair | |
D(1B) dopamine receptor
(Homo sapiens (Human)) | BDBM50010301
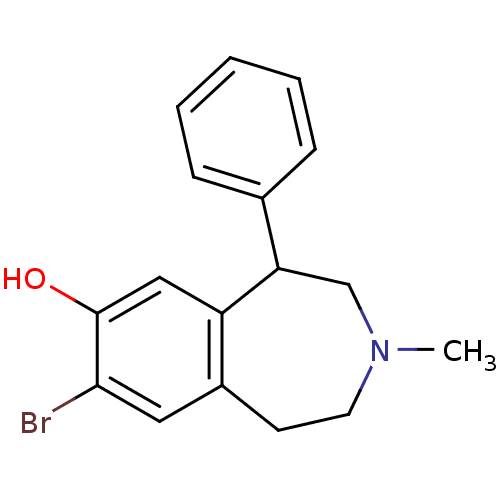
(8-Bromo-3-methyl-5-phenyl-2,3,4,5-tetrahydro-1H-be...)Show InChI InChI=1S/C17H18BrNO/c1-19-8-7-13-9-16(18)17(20)10-14(13)15(11-19)12-5-3-2-4-6-12/h2-6,9-10,15,20H,7-8,11H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]SCH23390 from dopamine D5 receptor (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128047
BindingDB Entry DOI: 10.7270/Q2CJ8J8S |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50170672

((2S,4aR,6aR,7R,9S,10aS,10bR)-methyl 2-(furan-3-yl)...)Show SMILES CCC(=O)O[C@H]1C[C@@H](C(=O)OC)[C@]2(C)CC[C@H]3C(=O)O[C@@H](C[C@]3(C)[C@H]2C1=O)c1ccoc1 |r| Show InChI InChI=1S/C24H30O8/c1-5-18(25)31-16-10-15(21(27)29-4)23(2)8-6-14-22(28)32-17(13-7-9-30-12-13)11-24(14,3)20(23)19(16)26/h7,9,12,14-17,20H,5-6,8,10-11H2,1-4H3/t14-,15-,16-,17-,20-,23-,24-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Iowa
Curated by ChEMBL
| Assay Description
Inhibition of [125I]- IOXY binding to human Opioid receptor kappa1 |
J Med Chem 48: 4765-71 (2005)
Article DOI: 10.1021/jm048963m
BindingDB Entry DOI: 10.7270/Q2DN44KP |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50159165
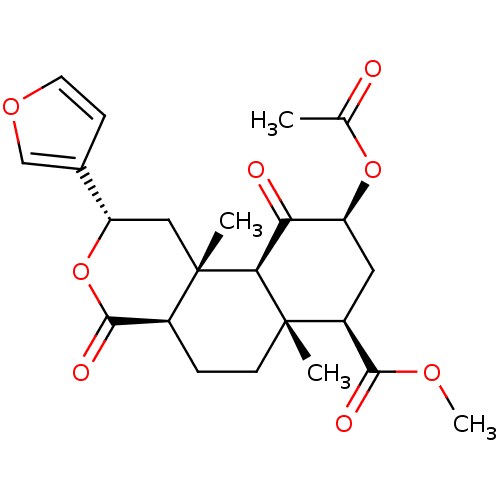
((2S,4aR,6aR,7R,9S,10aS,10bR)-9-(acetyloxy)-2-(fura...)Show SMILES COC(=O)[C@@H]1C[C@H](OC(C)=O)C(=O)[C@H]2[C@@]1(C)CC[C@H]1C(=O)O[C@@H](C[C@]21C)c1ccoc1 |r| Show InChI InChI=1S/C23H28O8/c1-12(24)30-16-9-15(20(26)28-4)22(2)7-5-14-21(27)31-17(13-6-8-29-11-13)10-23(14,3)19(22)18(16)25/h6,8,11,14-17,19H,5,7,9-10H2,1-4H3/t14-,15-,16-,17-,19-,22-,23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Iowa
Curated by ChEMBL
| Assay Description
Displacement of [125I]IOXY from human recombinant kappa opioid receptor expressed in CHO cells |
J Med Chem 51: 2421-31 (2008)
Article DOI: 10.1021/jm701162g
BindingDB Entry DOI: 10.7270/Q2DV1KR7 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50159165

((2S,4aR,6aR,7R,9S,10aS,10bR)-9-(acetyloxy)-2-(fura...)Show SMILES COC(=O)[C@@H]1C[C@H](OC(C)=O)C(=O)[C@H]2[C@@]1(C)CC[C@H]1C(=O)O[C@@H](C[C@]21C)c1ccoc1 |r| Show InChI InChI=1S/C23H28O8/c1-12(24)30-16-9-15(20(26)28-4)22(2)7-5-14-21(27)31-17(13-6-8-29-11-13)10-23(14,3)19(22)18(16)25/h6,8,11,14-17,19H,5,7,9-10H2,1-4H3/t14-,15-,16-,17-,19-,22-,23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Iowa
Curated by ChEMBL
| Assay Description
Displacement of [125]OXY from kappa opioid receptor |
J Nat Prod 69: 914-8 (2006)
Article DOI: 10.1021/np060094b
BindingDB Entry DOI: 10.7270/Q28W3D22 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50159165

((2S,4aR,6aR,7R,9S,10aS,10bR)-9-(acetyloxy)-2-(fura...)Show SMILES COC(=O)[C@@H]1C[C@H](OC(C)=O)C(=O)[C@H]2[C@@]1(C)CC[C@H]1C(=O)O[C@@H](C[C@]21C)c1ccoc1 |r| Show InChI InChI=1S/C23H28O8/c1-12(24)30-16-9-15(20(26)28-4)22(2)7-5-14-21(27)31-17(13-6-8-29-11-13)10-23(14,3)19(22)18(16)25/h6,8,11,14-17,19H,5,7,9-10H2,1-4H3/t14-,15-,16-,17-,19-,22-,23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Iowa
Curated by ChEMBL
| Assay Description
Inhibition of [125I]- IOXY binding to human Opioid receptor kappa1 |
J Med Chem 48: 4765-71 (2005)
Article DOI: 10.1021/jm048963m
BindingDB Entry DOI: 10.7270/Q2DN44KP |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50159165

((2S,4aR,6aR,7R,9S,10aS,10bR)-9-(acetyloxy)-2-(fura...)Show SMILES COC(=O)[C@@H]1C[C@H](OC(C)=O)C(=O)[C@H]2[C@@]1(C)CC[C@H]1C(=O)O[C@@H](C[C@]21C)c1ccoc1 |r| Show InChI InChI=1S/C23H28O8/c1-12(24)30-16-9-15(20(26)28-4)22(2)7-5-14-21(27)31-17(13-6-8-29-11-13)10-23(14,3)19(22)18(16)25/h6,8,11,14-17,19H,5,7,9-10H2,1-4H3/t14-,15-,16-,17-,19-,22-,23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Iowa
Curated by ChEMBL
| Assay Description
Agonist activity at human recombinant kappa opioid receptor expressed in CHO cells by [35S]GTP-gamma-S binding assay |
J Med Chem 51: 2421-31 (2008)
Article DOI: 10.1021/jm701162g
BindingDB Entry DOI: 10.7270/Q2DV1KR7 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM21398

(4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...)Show SMILES OC1(CCN(CCCC(=O)c2ccc(F)cc2)CC1)c1ccc(Cl)cc1 Show InChI InChI=1S/C21H23ClFNO2/c22-18-7-5-17(6-8-18)21(26)11-14-24(15-12-21)13-1-2-20(25)16-3-9-19(23)10-4-16/h3-10,26H,1-2,11-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
City University of New York
Curated by ChEMBL
| Assay Description
Binding affinity to human D2 receptor |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.127053
BindingDB Entry DOI: 10.7270/Q2H135KF |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Homo sapiens (Human)) | BDBM50160160
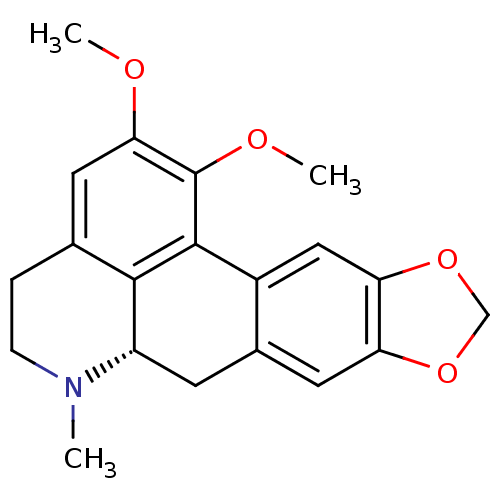
((S)-1,2-Dimethoxy-6-methyl-5,6,6a,7-tetrahydro-4H-...)Show SMILES COc1cc2CCN(C)[C@H]3Cc4cc5OCOc5cc4-c(c1OC)c23 Show InChI InChI=1S/C20H21NO4/c1-21-5-4-11-7-17(22-2)20(23-3)19-13-9-16-15(24-10-25-16)8-12(13)6-14(21)18(11)19/h7-9,14H,4-6,10H2,1-3H3/t14-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hunter College and the Graduate Center of the City University of New York
Curated by ChEMBL
| Assay Description
Displacement of [3H]Prazosin from human adrenergic alpha1A receptor |
Bioorg Med Chem Lett 20: 628-31 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.053
BindingDB Entry DOI: 10.7270/Q2C53MT3 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens) | BDBM50570806

(CHEMBL4876260)Show SMILES Oc1cc2CCN(Cc3ccccc3CNC(=O)c3cccc(Br)c3)Cc2cc1O | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-methylspiperone from dopamine D3 receptor (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128047
BindingDB Entry DOI: 10.7270/Q2CJ8J8S |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM50008735

((+)-3-(tert-butyl)-(3S,4aS,13bS)-2,3,4,4a,8,9,13b,...)Show SMILES CC(C)(C)[C@]1(O)CCN2C[C@H]3c4ccccc4CCc4cccc([C@@H]2C1)c34 Show InChI InChI=1S/C25H31NO/c1-24(2,3)25(27)13-14-26-16-21-19-9-5-4-7-17(19)11-12-18-8-6-10-20(23(18)21)22(26)15-25/h4-10,21-22,27H,11-16H2,1-3H3/t21-,22-,25-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
City University of New York
Curated by ChEMBL
| Assay Description
Binding affinity to human D1 receptor |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.127053
BindingDB Entry DOI: 10.7270/Q2H135KF |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50467922

(CHEMBL4283982)Show InChI InChI=1S/C21H25ClN2O3/c1-27-20-13-16-8-11-24(14-17(16)12-19(20)25)10-3-2-9-23-21(26)15-4-6-18(22)7-5-15/h4-7,12-13,25H,2-3,8-11,14H2,1H3,(H,23,26) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
City University of New York
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-Methylspiperone from human dopamine D3 receptor expressed in HEKT cell membranes |
ACS Med Chem Lett 9: 990-995 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00229
BindingDB Entry DOI: 10.7270/Q2T43WSN |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM50210764
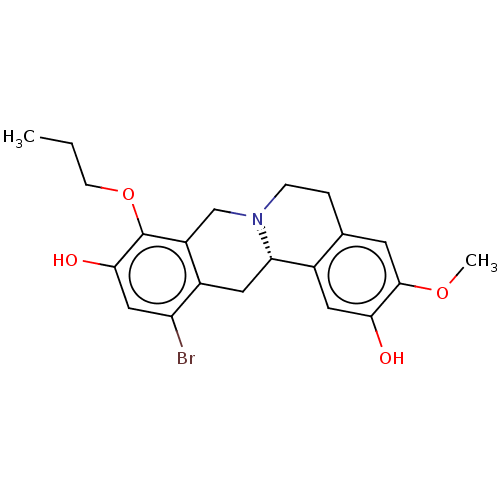
(CHEMBL3976659)Show SMILES [H][C@@]12Cc3c(Br)cc(O)c(OCCC)c3CN1CCc1cc(OC)c(O)cc21 |r| Show InChI InChI=1S/C21H24BrNO4/c1-3-6-27-21-15-11-23-5-4-12-7-20(26-2)18(24)9-13(12)17(23)8-14(15)16(22)10-19(21)25/h7,9-10,17,24-25H,3-6,8,11H2,1-2H3/t17-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
City University of New York
Curated by ChEMBL
| Assay Description
Displacement of [3H]SCH23390 from human D1 receptor expressed in HEKT cell membranes after 90 mins by microbeta scintillation counting method |
Eur J Med Chem 125: 255-268 (2017)
Article DOI: 10.1016/j.ejmech.2016.09.036
BindingDB Entry DOI: 10.7270/Q2KW5J1T |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM50210759
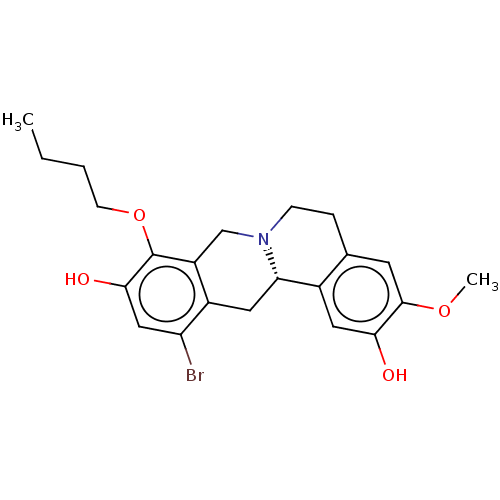
(CHEMBL3905947)Show SMILES [H][C@@]12Cc3c(Br)cc(O)c(OCCCC)c3CN1CCc1cc(OC)c(O)cc21 |r| Show InChI InChI=1S/C22H26BrNO4/c1-3-4-7-28-22-16-12-24-6-5-13-8-21(27-2)19(25)10-14(13)18(24)9-15(16)17(23)11-20(22)26/h8,10-11,18,25-26H,3-7,9,12H2,1-2H3/t18-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
City University of New York
Curated by ChEMBL
| Assay Description
Displacement of [3H]SCH23390 from human D1 receptor expressed in HEKT cell membranes after 90 mins by microbeta scintillation counting method |
Eur J Med Chem 125: 255-268 (2017)
Article DOI: 10.1016/j.ejmech.2016.09.036
BindingDB Entry DOI: 10.7270/Q2KW5J1T |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50170676
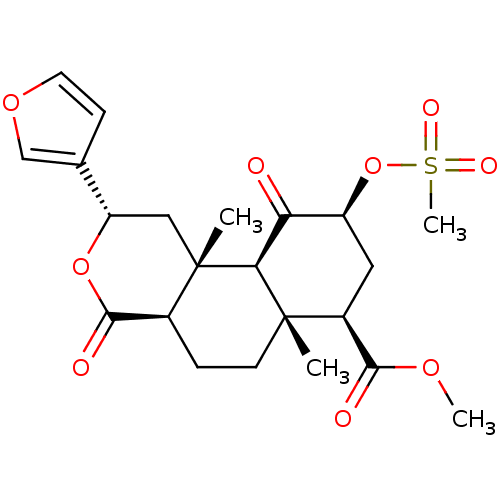
((2S,4aR,6aR,7R,9S,10aS,10bR)-methyl 2-(furan-3-yl)...)Show SMILES COC(=O)[C@@H]1C[C@H](OS(C)(=O)=O)C(=O)[C@H]2[C@@]1(C)CC[C@H]1C(=O)O[C@@H](C[C@]21C)c1ccoc1 |r| Show InChI InChI=1S/C22H28O9S/c1-21-7-5-13-20(25)30-16(12-6-8-29-11-12)10-22(13,2)18(21)17(23)15(31-32(4,26)27)9-14(21)19(24)28-3/h6,8,11,13-16,18H,5,7,9-10H2,1-4H3/t13-,14-,15-,16-,18-,21-,22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Iowa
Curated by ChEMBL
| Assay Description
Inhibition of [125I]- IOXY binding to human Opioid receptor kappa1 |
J Med Chem 48: 4765-71 (2005)
Article DOI: 10.1021/jm048963m
BindingDB Entry DOI: 10.7270/Q2DN44KP |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50170676

((2S,4aR,6aR,7R,9S,10aS,10bR)-methyl 2-(furan-3-yl)...)Show SMILES COC(=O)[C@@H]1C[C@H](OS(C)(=O)=O)C(=O)[C@H]2[C@@]1(C)CC[C@H]1C(=O)O[C@@H](C[C@]21C)c1ccoc1 |r| Show InChI InChI=1S/C22H28O9S/c1-21-7-5-13-20(25)30-16(12-6-8-29-11-12)10-22(13,2)18(21)17(23)15(31-32(4,26)27)9-14(21)19(24)28-3/h6,8,11,13-16,18H,5,7,9-10H2,1-4H3/t13-,14-,15-,16-,18-,21-,22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Iowa
Curated by ChEMBL
| Assay Description
Displacement of [125]OXY from kappa opioid receptor |
J Nat Prod 69: 914-8 (2006)
Article DOI: 10.1021/np060094b
BindingDB Entry DOI: 10.7270/Q28W3D22 |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM50008735

((+)-3-(tert-butyl)-(3S,4aS,13bS)-2,3,4,4a,8,9,13b,...)Show SMILES CC(C)(C)[C@]1(O)CCN2C[C@H]3c4ccccc4CCc4cccc([C@@H]2C1)c34 Show InChI InChI=1S/C25H31NO/c1-24(2,3)25(27)13-14-26-16-21-19-9-5-4-7-17(19)11-12-18-8-6-10-20(23(18)21)22(26)15-25/h4-10,21-22,27H,11-16H2,1-3H3/t21-,22-,25-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
City University of New York
Curated by ChEMBL
| Assay Description
Displacement of [3H]SCH2390 from human dopamine D1 receptor by PDSP assay |
Bioorg Med Chem 24: 2060-71 (2016)
Article DOI: 10.1016/j.bmc.2016.03.037
BindingDB Entry DOI: 10.7270/Q2SX6G3G |
More data for this
Ligand-Target Pair | |
D(1B) dopamine receptor
(Homo sapiens (Human)) | BDBM50010301

(8-Bromo-3-methyl-5-phenyl-2,3,4,5-tetrahydro-1H-be...)Show InChI InChI=1S/C17H18BrNO/c1-19-8-7-13-9-16(18)17(20)10-14(13)15(11-19)12-5-3-2-4-6-12/h2-6,9-10,15,20H,7-8,11H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
City University of New York
Curated by ChEMBL
| Assay Description
Binding affinity to human D5 receptor |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.127053
BindingDB Entry DOI: 10.7270/Q2H135KF |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50487259

(CHEBI:64219 | [3H]NEMONAPRIDE)Show SMILES CNc1cc(OC)c(cc1Cl)C(=O)N[C@@H]1CCN(Cc2ccccc2)[C@@H]1C |r| Show InChI InChI=1S/C21H26ClN3O2/c1-14-18(9-10-25(14)13-15-7-5-4-6-8-15)24-21(26)16-11-17(22)19(23-2)12-20(16)27-3/h4-8,11-12,14,18,23H,9-10,13H2,1-3H3,(H,24,26)/t14-,18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]N-methylspiperone from dopamine D2 receptor (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128047
BindingDB Entry DOI: 10.7270/Q2CJ8J8S |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50467924
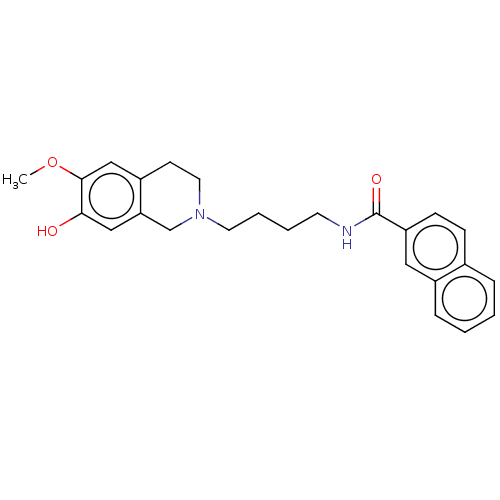
(CHEMBL4287062)Show SMILES COc1cc2CCN(CCCCNC(=O)c3ccc4ccccc4c3)Cc2cc1O Show InChI InChI=1S/C25H28N2O3/c1-30-24-16-20-10-13-27(17-22(20)15-23(24)28)12-5-4-11-26-25(29)21-9-8-18-6-2-3-7-19(18)14-21/h2-3,6-9,14-16,28H,4-5,10-13,17H2,1H3,(H,26,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
City University of New York
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-Methylspiperone from human dopamine D3 receptor expressed in HEKT cell membranes |
ACS Med Chem Lett 9: 990-995 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00229
BindingDB Entry DOI: 10.7270/Q2T43WSN |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50467929
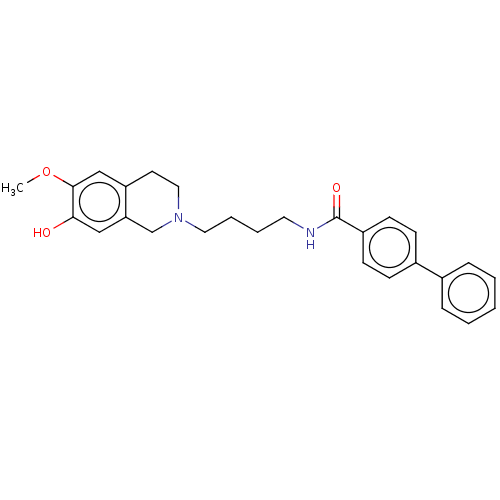
(CHEMBL4287471)Show SMILES COc1cc2CCN(CCCCNC(=O)c3ccc(cc3)-c3ccccc3)Cc2cc1O Show InChI InChI=1S/C27H30N2O3/c1-32-26-18-23-13-16-29(19-24(23)17-25(26)30)15-6-5-14-28-27(31)22-11-9-21(10-12-22)20-7-3-2-4-8-20/h2-4,7-12,17-18,30H,5-6,13-16,19H2,1H3,(H,28,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
City University of New York
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-Methylspiperone from human dopamine D3 receptor expressed in HEKT cell membranes |
ACS Med Chem Lett 9: 990-995 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00229
BindingDB Entry DOI: 10.7270/Q2T43WSN |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM50366495

((+)butaclamol | CHEMBL1255588)Show SMILES CC(C)(C)[C@@]1(O)CCN2C[C@@H]3c4ccccc4CCc4cccc([C@H]2C1)c34 |r| Show InChI InChI=1S/C25H31NO/c1-24(2,3)25(27)13-14-26-16-21-19-9-5-4-7-17(19)11-12-18-8-6-10-20(23(18)21)22(26)15-25/h4-10,21-22,27H,11-16H2,1-3H3/t21-,22-,25-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]SCH23390 from dopamine D1 receptor (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128047
BindingDB Entry DOI: 10.7270/Q2CJ8J8S |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50001884

(2-[4-(4-Methyl-benzyl)-piperazin-1-yl]-1-(2-methyl...)Show SMILES CN1CCN(CC1)C1=Nc2cc(Cl)ccc2Nc2ccccc12 |t:8| Show InChI InChI=1S/C18H19ClN4/c1-22-8-10-23(11-9-22)18-14-4-2-3-5-15(14)20-16-7-6-13(19)12-17(16)21-18/h2-7,12,20H,8-11H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
City University of New York
Curated by ChEMBL
| Assay Description
Binding affinity to human 5HT2A receptor |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.127053
BindingDB Entry DOI: 10.7270/Q2H135KF |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50216132
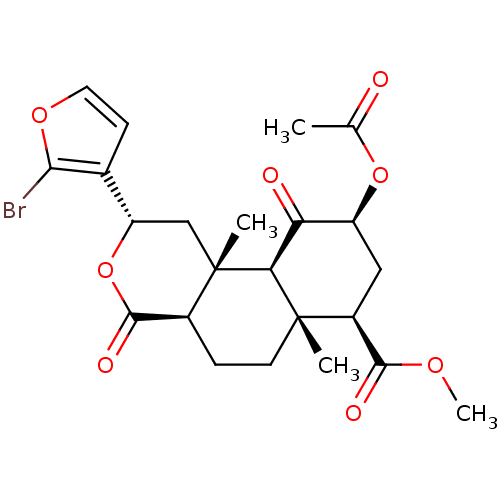
((2S,4aR,6aR,7R,9S,10aS,10bR)-methyl 9-acetoxy-2-(2...)Show SMILES COC(=O)[C@@H]1C[C@H](OC(C)=O)C(=O)[C@H]2[C@@]1(C)CC[C@H]1C(=O)O[C@@H](C[C@]21C)c1ccoc1Br |r| Show InChI InChI=1S/C23H27BrO8/c1-11(25)31-15-9-14(20(27)29-4)22(2)7-5-13-21(28)32-16(12-6-8-30-19(12)24)10-23(13,3)18(22)17(15)26/h6,8,13-16,18H,5,7,9-10H2,1-4H3/t13-,14-,15-,16-,18-,22-,23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Iowa
Curated by ChEMBL
| Assay Description
Displacement of [125I]IOXY from human kappa opioid receptor expressed in CHO cells |
J Med Chem 50: 3596-603 (2007)
Article DOI: 10.1021/jm070393d
BindingDB Entry DOI: 10.7270/Q2ZK5HH0 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50001884

(2-[4-(4-Methyl-benzyl)-piperazin-1-yl]-1-(2-methyl...)Show SMILES CN1CCN(CC1)C1=Nc2cc(Cl)ccc2Nc2ccccc12 |t:8| Show InChI InChI=1S/C18H19ClN4/c1-22-8-10-23(11-9-22)18-14-4-2-3-5-15(14)20-16-7-6-13(19)12-17(16)21-18/h2-7,12,20H,8-11H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
City University of New York
Curated by ChEMBL
| Assay Description
Displacement of [3H]-Ketanserin from 5-HT2A receptor (unknown origin) incubated for 90 mins by microbeta counting method |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2020.115578
BindingDB Entry DOI: 10.7270/Q22B92KJ |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50376845
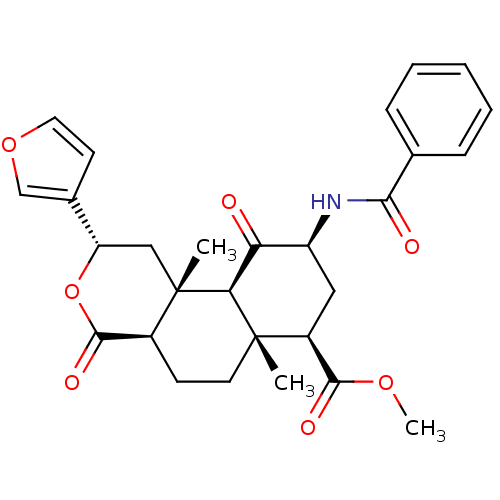
(CHEMBL259658)Show SMILES COC(=O)[C@@H]1C[C@H](NC(=O)c2ccccc2)C(=O)[C@H]2[C@@]1(C)CC[C@H]1C(=O)O[C@@H](C[C@]21C)c1ccoc1 Show InChI InChI=1S/C28H31NO7/c1-27-11-9-18-26(33)36-21(17-10-12-35-15-17)14-28(18,2)23(27)22(30)20(13-19(27)25(32)34-3)29-24(31)16-7-5-4-6-8-16/h4-8,10,12,15,18-21,23H,9,11,13-14H2,1-3H3,(H,29,31)/t18-,19-,20-,21-,23-,27-,28-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Iowa
Curated by ChEMBL
| Assay Description
Displacement of [125I]IOXY from human recombinant mu opioid receptor expressed in CHO cells |
J Med Chem 51: 2421-31 (2008)
Article DOI: 10.1021/jm701162g
BindingDB Entry DOI: 10.7270/Q2DV1KR7 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(Homo sapiens (Human)) | BDBM50178979
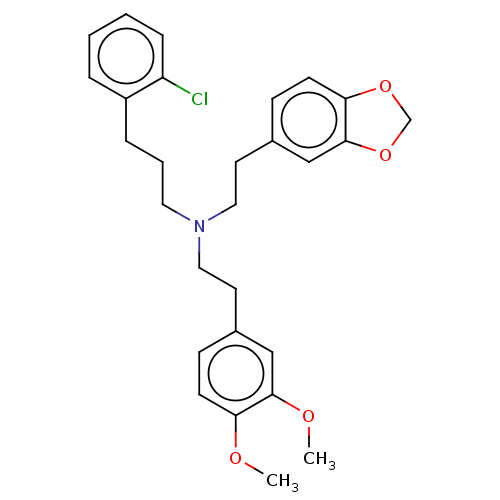
(CHEMBL3813796)Show SMILES COc1ccc(CCN(CCCc2ccccc2Cl)CCc2ccc3OCOc3c2)cc1OC Show InChI InChI=1S/C28H32ClNO4/c1-31-25-11-9-21(18-27(25)32-2)13-16-30(15-5-7-23-6-3-4-8-24(23)29)17-14-22-10-12-26-28(19-22)34-20-33-26/h3-4,6,8-12,18-19H,5,7,13-17,20H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
City University Of New York
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-HT2B receptor measured after 90 mins by microbeta scintillation counting method |
Bioorg Med Chem Lett 26: 3216-3219 (2016)
Article DOI: 10.1016/j.bmcl.2016.05.079
BindingDB Entry DOI: 10.7270/Q29S1SXB |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(Homo sapiens (Human)) | BDBM50178980

(CHEMBL3813726)Show SMILES COc1ccc(CCN(CCCc2ccccc2Br)CCc2ccc3OCOc3c2)cc1OC Show InChI InChI=1S/C28H32BrNO4/c1-31-25-11-9-21(18-27(25)32-2)13-16-30(15-5-7-23-6-3-4-8-24(23)29)17-14-22-10-12-26-28(19-22)34-20-33-26/h3-4,6,8-12,18-19H,5,7,13-17,20H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
City University Of New York
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-HT2B receptor measured after 90 mins by microbeta scintillation counting method |
Bioorg Med Chem Lett 26: 3216-3219 (2016)
Article DOI: 10.1016/j.bmcl.2016.05.079
BindingDB Entry DOI: 10.7270/Q29S1SXB |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50467928
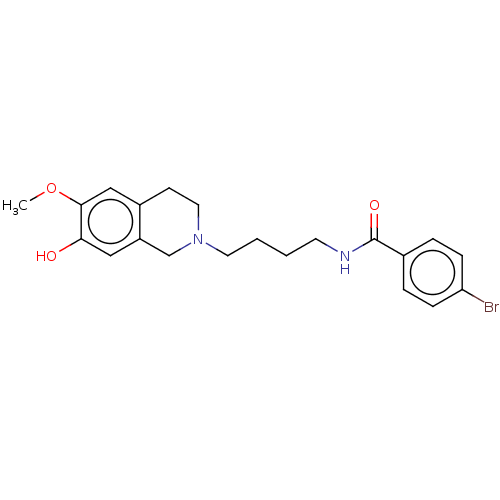
(CHEMBL4291172)Show InChI InChI=1S/C21H25BrN2O3/c1-27-20-13-16-8-11-24(14-17(16)12-19(20)25)10-3-2-9-23-21(26)15-4-6-18(22)7-5-15/h4-7,12-13,25H,2-3,8-11,14H2,1H3,(H,23,26) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
City University of New York
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-Methylspiperone from human dopamine D3 receptor expressed in HEKT cell membranes |
ACS Med Chem Lett 9: 990-995 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00229
BindingDB Entry DOI: 10.7270/Q2T43WSN |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens) | BDBM50570809
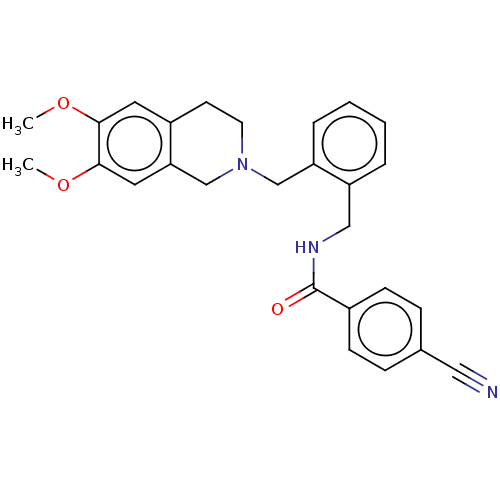
(CHEMBL4878587)Show SMILES COc1cc2CCN(Cc3ccccc3CNC(=O)c3ccc(cc3)C#N)Cc2cc1OC | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-methylspiperone from dopamine D3 receptor (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128047
BindingDB Entry DOI: 10.7270/Q2CJ8J8S |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50371092
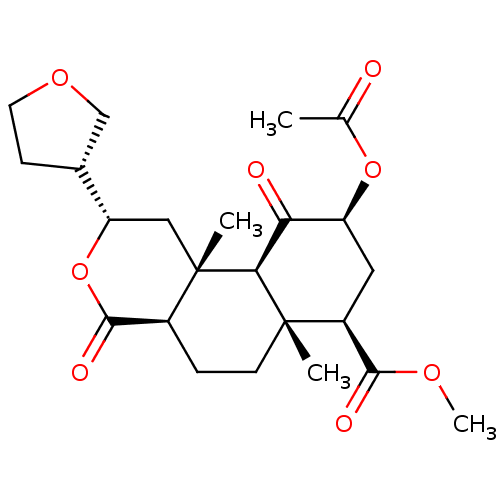
(CHEMBL427280)Show SMILES COC(=O)[C@@H]1C[C@H](OC(C)=O)C(=O)[C@H]2[C@@]1(C)CC[C@H]1C(=O)O[C@@H](C[C@]21C)[C@@H]1CCOC1 Show InChI InChI=1S/C23H32O8/c1-12(24)30-16-9-15(20(26)28-4)22(2)7-5-14-21(27)31-17(13-6-8-29-11-13)10-23(14,3)19(22)18(16)25/h13-17,19H,5-11H2,1-4H3/t13-,14+,15+,16+,17+,19+,22+,23+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Iowa
Curated by ChEMBL
| Assay Description
Displacement of [125I]IOXY from human kappa opioid receptor expressed in CHO cells |
J Med Chem 50: 3596-603 (2007)
Article DOI: 10.1021/jm070393d
BindingDB Entry DOI: 10.7270/Q2ZK5HH0 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data