| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Cholinesterase |
|---|
| Ligand | BDBM50149956 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_1558460 (CHEMBL3773336) |
|---|
| IC50 | 90320±n/a nM |
|---|
| Citation |  Pejchal, V; ?tepánková, ?; Pejchalová, M; Královec, K; Havelek, R; Ru?icková, Z; Ajani, H; Lo, R; Lep?ík, M Synthesis, structural characterization, docking, lipophilicity and cytotoxicity of 1-[(1R)-1-(6-fluoro-1,3-benzothiazol-2-yl)ethyl]-3-alkyl carbamates, novel acetylcholinesterase and butyrylcholinesterase pseudo-irreversible inhibitors. Bioorg Med Chem24:1560-72 (2016) [PubMed] Article Pejchal, V; ?tepánková, ?; Pejchalová, M; Královec, K; Havelek, R; Ru?icková, Z; Ajani, H; Lo, R; Lep?ík, M Synthesis, structural characterization, docking, lipophilicity and cytotoxicity of 1-[(1R)-1-(6-fluoro-1,3-benzothiazol-2-yl)ethyl]-3-alkyl carbamates, novel acetylcholinesterase and butyrylcholinesterase pseudo-irreversible inhibitors. Bioorg Med Chem24:1560-72 (2016) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Cholinesterase |
|---|
| Name: | Cholinesterase |
|---|
| Synonyms: | BCHE | Butyrylcholinesterase (BuChE) | CHLE_HORSE | Cholinesterase |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 65643.35 |
|---|
| Organism: | Equus caballus (Horse) |
|---|
| Description: | P81908 |
|---|
| Residue: | 574 |
|---|
| Sequence: | EEDIIITTKNGKVRGMNLPVLGGTVTAFLGIPYAQPPLGRLRFKKPQSLTKWSNIWNATK
YANSCYQNTDQSFPGFLGSEMWNPNTELSEDCLYLNVWIPAPKPKNATVMIWIYGGGFQT
GTSSLPVYDGKFLARVERVIVVSMNYRVGALGFLALSENPEAPGNMGLFDQQLALQWVQK
NIAAFGGNPRSVTLFGESAGAASVSLHLLSPRSQPLFTRAILQSGSSNAPWAVTSLYEAR
NRTLTLAKRMGCSRDNETEMIKCLRDKDPQEILLNEVFVVPYDTLLSVNFGPTVDGDFLT
DMPDTLLQLGQFKRTQILVGVNKDEGTAFLVYGAPGFSKDNNSIITRKEFQEGLKIFFPR
VSEFGRESILFHYMDWLDDQRAENYREALDDVVGDYNIICPALEFTRKFSELGNDAFFYY
FEHRSTKLPWPEWMGVMHGYEIEFVFGLPLERRVNYTRAEEILSRSIMKRWANFAKYGNP
NGTQNNSTRWPVFKSTEQKYLTLNTESPKVYTKLRAQQCRFWTLFFPKVLELTGNIDEAE
REWKAGFHRWNNYMMDWKNQFNDYTSKKESCSDF
|
|
|
|---|
| BDBM50149956 |
|---|
| n/a |
|---|
| Name | BDBM50149956 |
|---|
| Synonyms: | CHEMBL3770401 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C11H11FN2O2S |
|---|
| Mol. Mass. | 254.281 |
|---|
| SMILES | COC(=O)N[C@H](C)c1nc2ccc(F)cc2s1 |r| |
|---|
| Structure | 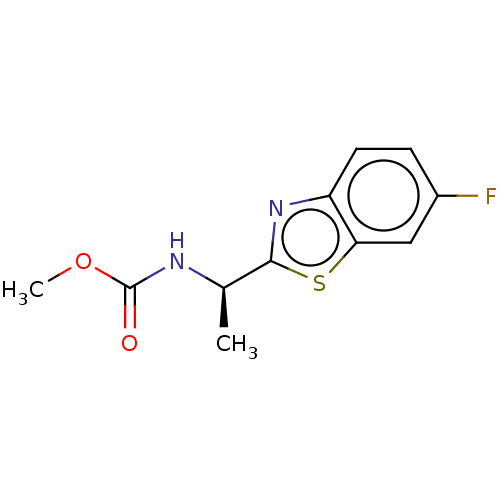
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Pejchal, V; ?tepánková, ?; Pejchalová, M; Královec, K; Havelek, R; Ru?icková, Z; Ajani, H; Lo, R; Lep?ík, M Synthesis, structural characterization, docking, lipophilicity and cytotoxicity of 1-[(1R)-1-(6-fluoro-1,3-benzothiazol-2-yl)ethyl]-3-alkyl carbamates, novel acetylcholinesterase and butyrylcholinesterase pseudo-irreversible inhibitors. Bioorg Med Chem24:1560-72 (2016) [PubMed] Article
Pejchal, V; ?tepánková, ?; Pejchalová, M; Královec, K; Havelek, R; Ru?icková, Z; Ajani, H; Lo, R; Lep?ík, M Synthesis, structural characterization, docking, lipophilicity and cytotoxicity of 1-[(1R)-1-(6-fluoro-1,3-benzothiazol-2-yl)ethyl]-3-alkyl carbamates, novel acetylcholinesterase and butyrylcholinesterase pseudo-irreversible inhibitors. Bioorg Med Chem24:1560-72 (2016) [PubMed] Article