| Reaction Details |
|---|
 | Report a problem with these data |
| Target | 5'-nucleotidase |
|---|
| Ligand | BDBM50165146 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_1572756 (CHEMBL3804265) |
|---|
| Ki | 172±n/a nM |
|---|
| Citation |  al-Rashida, M; Batool, G; Sattar, A; Ejaz, SA; Khan, S; Lecka, J; Sévigny, J; Hameed, A; Iqbal, J 2-Alkoxy-3-(sulfonylarylaminomethylene)-chroman-4-ones as potent and selective inhibitors of ectonucleotidases. Eur J Med Chem115:484-94 (2016) [PubMed] Article al-Rashida, M; Batool, G; Sattar, A; Ejaz, SA; Khan, S; Lecka, J; Sévigny, J; Hameed, A; Iqbal, J 2-Alkoxy-3-(sulfonylarylaminomethylene)-chroman-4-ones as potent and selective inhibitors of ectonucleotidases. Eur J Med Chem115:484-94 (2016) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| 5'-nucleotidase |
|---|
| Name: | 5'-nucleotidase |
|---|
| Synonyms: | 5'-nucleotidase | 5NTD_RAT | Ecto-5'-nucleotidase (e5'NT) | Ecto-5-nucleotidase (e5'NT) | NT | Nt5 | Nt5e | Nte |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 63971.44 |
|---|
| Organism: | Rattus norvegicus (Rat) |
|---|
| Description: | P21588 |
|---|
| Residue: | 576 |
|---|
| Sequence: | MRPAAATAPKWLLLALSALLPLWPTAKSWELTIMHTNDVHSRLEQTSDDSTKCLNASLCV
GGVARLFTKVQQIRKEEPNVLLLDAGDQYQGTIWFTVYKGLEVAHFMNLLGYDAMALGNH
EFDNGVEGLIDPLLRNVKFPILSANIKARGPLAPQISGLYLPYKVLSVGGEVVGIVGYTS
KETPFLSNPGTNLVFEDEVTALQPEVDKLKTLNVNKIIALGHSGFEMDKLIAQKVRGVDV
VVGGHTNTFLYTGNPPSKEVPAGKYPFIVTSDDGRKVPVVQAYAFGKYLGYLKVEFDDKG
NVVTSYGNPILLNSTIREDAAIKADINQWRIKLDNYSTQELGRTIVYLNGSAQECRFREC
NMGNLICDAMINNNLRHPDEMFWNHVSMCIVNGGGIRSPIDERNNGTITWENLAAVLPFG
GTFDLVQLKGSTLKKAFEHSVHRYGQSTGEFLQVGGIHVVYDISRKPWDRVVQLKVLCTK
CRVPIYEPLEMDKVYKVVLPSYLVNGGDGFQMIKDELLKHDSGDQDISVVSEYISKMKVI
YPAVEGRIKFSAASHYQGSFPLIILSFWAVILVLYQ
|
|
|
|---|
| BDBM50165146 |
|---|
| n/a |
|---|
| Name | BDBM50165146 |
|---|
| Synonyms: | CHEMBL3798795 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C20H22N2O5S |
|---|
| Mol. Mass. | 402.464 |
|---|
| SMILES | CCCCOC1Oc2ccccc2C(=O)\C1=C/Nc1cccc(c1)S(N)(=O)=O |
|---|
| Structure | 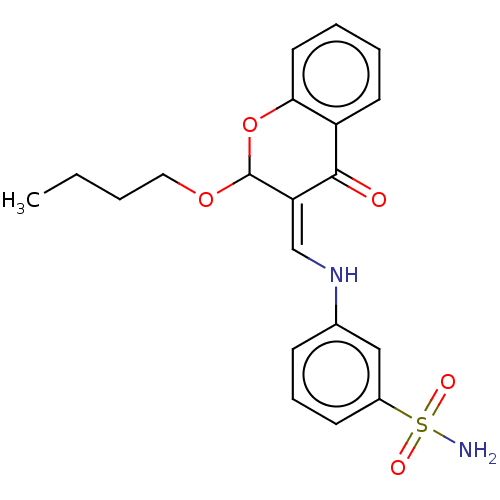
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 al-Rashida, M; Batool, G; Sattar, A; Ejaz, SA; Khan, S; Lecka, J; Sévigny, J; Hameed, A; Iqbal, J 2-Alkoxy-3-(sulfonylarylaminomethylene)-chroman-4-ones as potent and selective inhibitors of ectonucleotidases. Eur J Med Chem115:484-94 (2016) [PubMed] Article
al-Rashida, M; Batool, G; Sattar, A; Ejaz, SA; Khan, S; Lecka, J; Sévigny, J; Hameed, A; Iqbal, J 2-Alkoxy-3-(sulfonylarylaminomethylene)-chroman-4-ones as potent and selective inhibitors of ectonucleotidases. Eur J Med Chem115:484-94 (2016) [PubMed] Article