

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50143813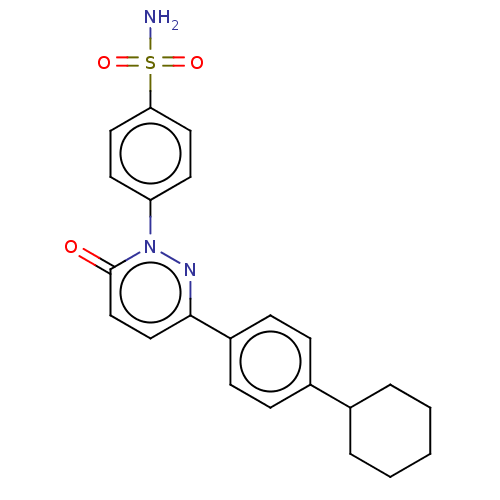 (CHEMBL3759894) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.980 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard (Hamdard University) Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase-2 using p-nitrophenyl acetate as substrate by esterase assay | Bioorg Med Chem Lett 26: 1337-41 (2016) Article DOI: 10.1016/j.bmcl.2015.12.016 BindingDB Entry DOI: 10.7270/Q2RV0QJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50143808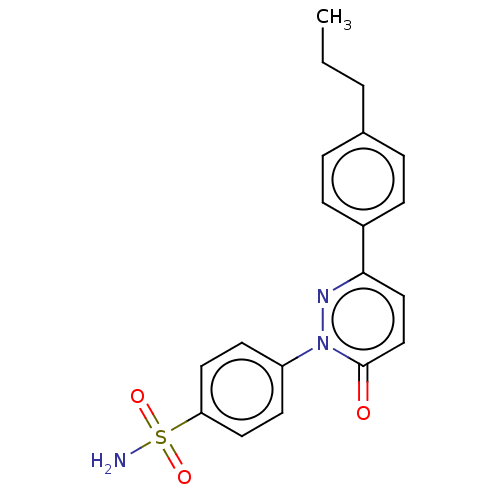 (CHEMBL3759134) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard (Hamdard University) Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase-1 using p-nitrophenyl acetate as substrate by esterase assay | Bioorg Med Chem Lett 26: 1337-41 (2016) Article DOI: 10.1016/j.bmcl.2015.12.016 BindingDB Entry DOI: 10.7270/Q2RV0QJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50143812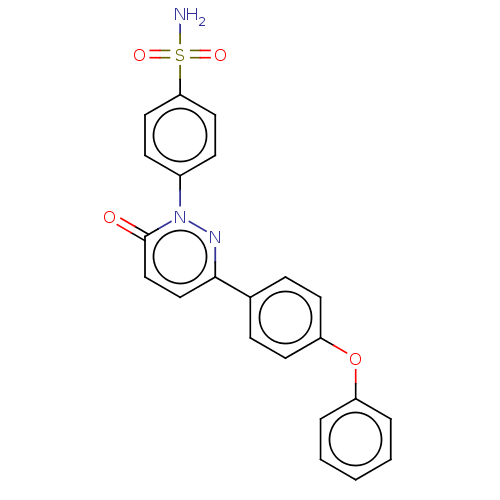 (CHEMBL3760118) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard (Hamdard University) Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase-2 using p-nitrophenyl acetate as substrate by esterase assay | Bioorg Med Chem Lett 26: 1337-41 (2016) Article DOI: 10.1016/j.bmcl.2015.12.016 BindingDB Entry DOI: 10.7270/Q2RV0QJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50143812 (CHEMBL3760118) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard (Hamdard University) Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase-1 using p-nitrophenyl acetate as substrate by esterase assay | Bioorg Med Chem Lett 26: 1337-41 (2016) Article DOI: 10.1016/j.bmcl.2015.12.016 BindingDB Entry DOI: 10.7270/Q2RV0QJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50143814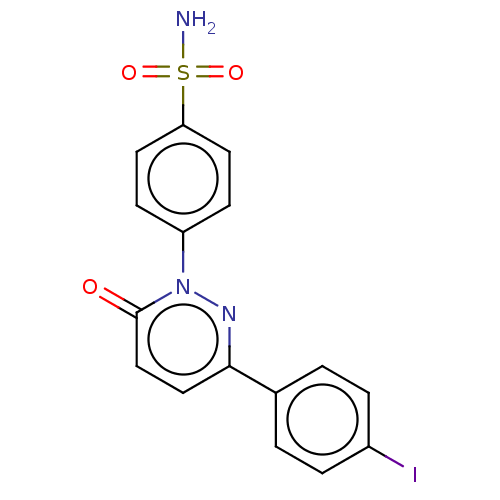 (CHEMBL3758784) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard (Hamdard University) Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase-2 using p-nitrophenyl acetate as substrate by esterase assay | Bioorg Med Chem Lett 26: 1337-41 (2016) Article DOI: 10.1016/j.bmcl.2015.12.016 BindingDB Entry DOI: 10.7270/Q2RV0QJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50143807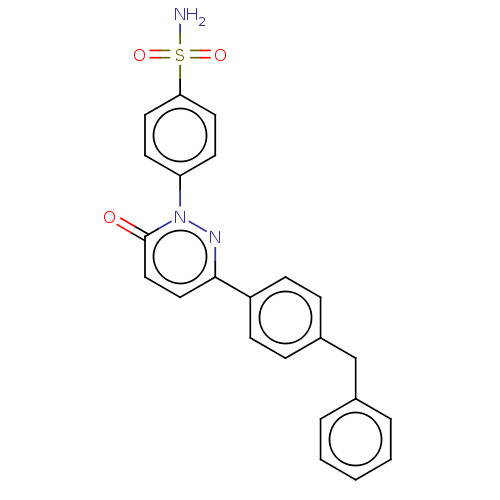 (CHEMBL3758698) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard (Hamdard University) Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase-2 using p-nitrophenyl acetate as substrate by esterase assay | Bioorg Med Chem Lett 26: 1337-41 (2016) Article DOI: 10.1016/j.bmcl.2015.12.016 BindingDB Entry DOI: 10.7270/Q2RV0QJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50143810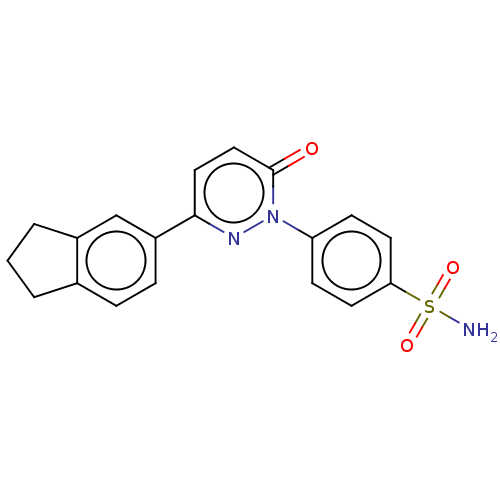 (CHEMBL3758504) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard (Hamdard University) Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase-2 using p-nitrophenyl acetate as substrate by esterase assay | Bioorg Med Chem Lett 26: 1337-41 (2016) Article DOI: 10.1016/j.bmcl.2015.12.016 BindingDB Entry DOI: 10.7270/Q2RV0QJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50143806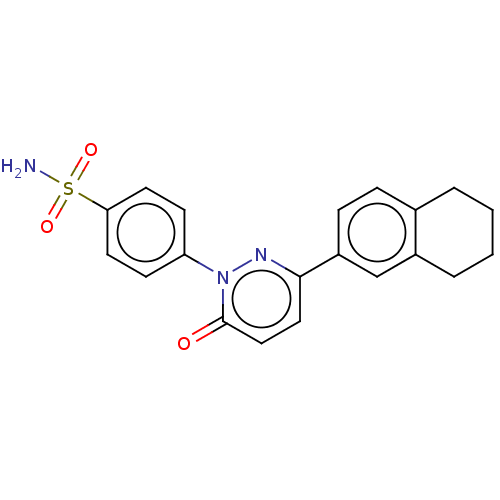 (CHEMBL3760111) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard (Hamdard University) Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase-2 using p-nitrophenyl acetate as substrate by esterase assay | Bioorg Med Chem Lett 26: 1337-41 (2016) Article DOI: 10.1016/j.bmcl.2015.12.016 BindingDB Entry DOI: 10.7270/Q2RV0QJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50143811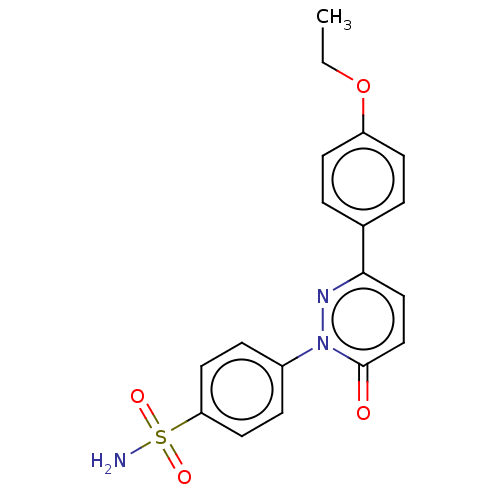 (CHEMBL3759464) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard (Hamdard University) Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase-1 using p-nitrophenyl acetate as substrate by esterase assay | Bioorg Med Chem Lett 26: 1337-41 (2016) Article DOI: 10.1016/j.bmcl.2015.12.016 BindingDB Entry DOI: 10.7270/Q2RV0QJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50143815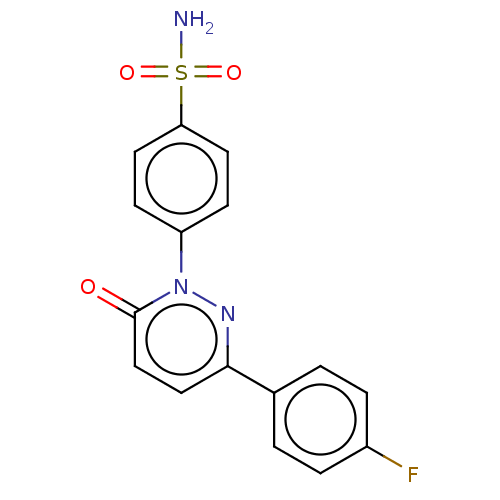 (CHEMBL3759883) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard (Hamdard University) Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase-2 using p-nitrophenyl acetate as substrate by esterase assay | Bioorg Med Chem Lett 26: 1337-41 (2016) Article DOI: 10.1016/j.bmcl.2015.12.016 BindingDB Entry DOI: 10.7270/Q2RV0QJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50143809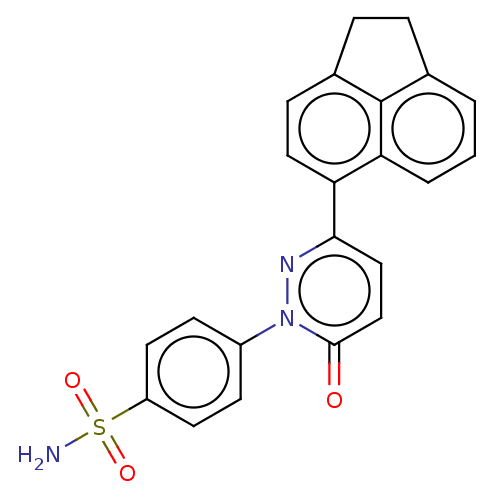 (CHEMBL3759757) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard (Hamdard University) Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase-1 using p-nitrophenyl acetate as substrate by esterase assay | Bioorg Med Chem Lett 26: 1337-41 (2016) Article DOI: 10.1016/j.bmcl.2015.12.016 BindingDB Entry DOI: 10.7270/Q2RV0QJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50143809 (CHEMBL3759757) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard (Hamdard University) Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase-2 using p-nitrophenyl acetate as substrate by esterase assay | Bioorg Med Chem Lett 26: 1337-41 (2016) Article DOI: 10.1016/j.bmcl.2015.12.016 BindingDB Entry DOI: 10.7270/Q2RV0QJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50143808 (CHEMBL3759134) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard (Hamdard University) Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase-2 using p-nitrophenyl acetate as substrate by esterase assay | Bioorg Med Chem Lett 26: 1337-41 (2016) Article DOI: 10.1016/j.bmcl.2015.12.016 BindingDB Entry DOI: 10.7270/Q2RV0QJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50143811 (CHEMBL3759464) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard (Hamdard University) Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase-2 using p-nitrophenyl acetate as substrate by esterase assay | Bioorg Med Chem Lett 26: 1337-41 (2016) Article DOI: 10.1016/j.bmcl.2015.12.016 BindingDB Entry DOI: 10.7270/Q2RV0QJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50143814 (CHEMBL3758784) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard (Hamdard University) Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase-1 using p-nitrophenyl acetate as substrate by esterase assay | Bioorg Med Chem Lett 26: 1337-41 (2016) Article DOI: 10.1016/j.bmcl.2015.12.016 BindingDB Entry DOI: 10.7270/Q2RV0QJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50143816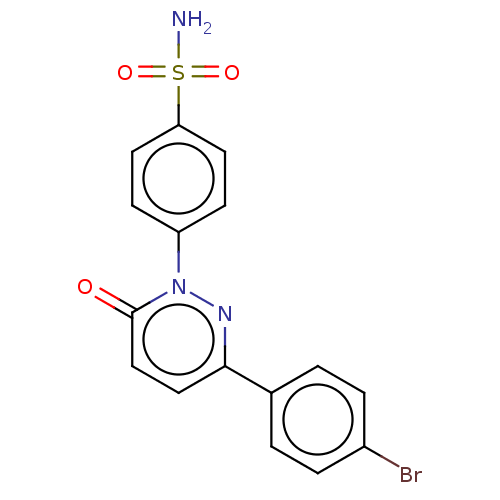 (CHEMBL3758388) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard (Hamdard University) Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase-2 using p-nitrophenyl acetate as substrate by esterase assay | Bioorg Med Chem Lett 26: 1337-41 (2016) Article DOI: 10.1016/j.bmcl.2015.12.016 BindingDB Entry DOI: 10.7270/Q2RV0QJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50143815 (CHEMBL3759883) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard (Hamdard University) Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase-1 using p-nitrophenyl acetate as substrate by esterase assay | Bioorg Med Chem Lett 26: 1337-41 (2016) Article DOI: 10.1016/j.bmcl.2015.12.016 BindingDB Entry DOI: 10.7270/Q2RV0QJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50143810 (CHEMBL3758504) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard (Hamdard University) Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase-1 using p-nitrophenyl acetate as substrate by esterase assay | Bioorg Med Chem Lett 26: 1337-41 (2016) Article DOI: 10.1016/j.bmcl.2015.12.016 BindingDB Entry DOI: 10.7270/Q2RV0QJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50143805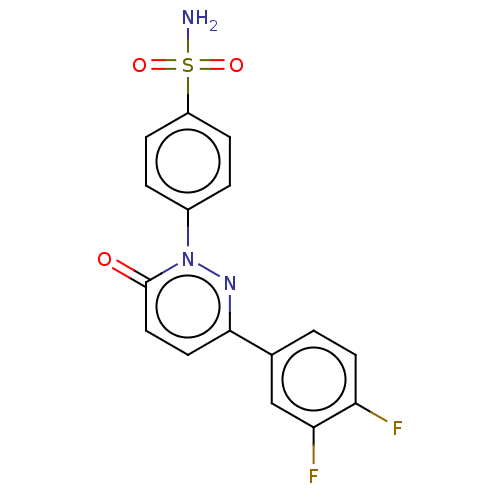 (CHEMBL3759344) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard (Hamdard University) Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase-1 using p-nitrophenyl acetate as substrate by esterase assay | Bioorg Med Chem Lett 26: 1337-41 (2016) Article DOI: 10.1016/j.bmcl.2015.12.016 BindingDB Entry DOI: 10.7270/Q2RV0QJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Rattus norvegicus (Rat)) | BDBM50437933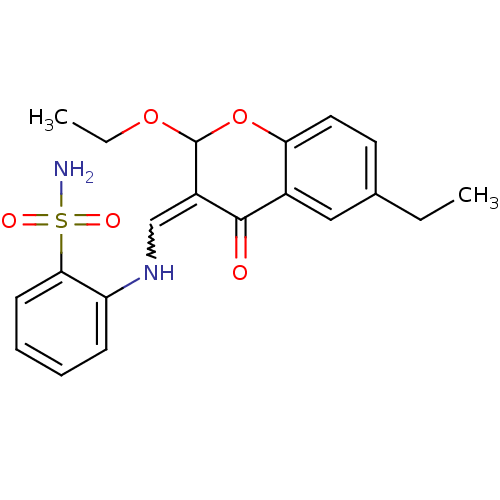 (CHEMBL2408703) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Forman Christian College (A Chartered University) Curated by ChEMBL | Assay Description Inhibition of rat Ecto-5'-nucleotidase transfected in COS7 cells preincubated for 10 mins followed by AMP addition measured after 10 mins | Eur J Med Chem 115: 484-94 (2016) Article DOI: 10.1016/j.ejmech.2016.02.073 BindingDB Entry DOI: 10.7270/Q2K939D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50143813 (CHEMBL3759894) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard (Hamdard University) Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase-1 using p-nitrophenyl acetate as substrate by esterase assay | Bioorg Med Chem Lett 26: 1337-41 (2016) Article DOI: 10.1016/j.bmcl.2015.12.016 BindingDB Entry DOI: 10.7270/Q2RV0QJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50143806 (CHEMBL3760111) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard (Hamdard University) Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase-1 using p-nitrophenyl acetate as substrate by esterase assay | Bioorg Med Chem Lett 26: 1337-41 (2016) Article DOI: 10.1016/j.bmcl.2015.12.016 BindingDB Entry DOI: 10.7270/Q2RV0QJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50143805 (CHEMBL3759344) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard (Hamdard University) Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase-2 using p-nitrophenyl acetate as substrate by esterase assay | Bioorg Med Chem Lett 26: 1337-41 (2016) Article DOI: 10.1016/j.bmcl.2015.12.016 BindingDB Entry DOI: 10.7270/Q2RV0QJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50143816 (CHEMBL3758388) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard (Hamdard University) Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase-1 using p-nitrophenyl acetate as substrate by esterase assay | Bioorg Med Chem Lett 26: 1337-41 (2016) Article DOI: 10.1016/j.bmcl.2015.12.016 BindingDB Entry DOI: 10.7270/Q2RV0QJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50143807 (CHEMBL3758698) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard (Hamdard University) Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase-1 using p-nitrophenyl acetate as substrate by esterase assay | Bioorg Med Chem Lett 26: 1337-41 (2016) Article DOI: 10.1016/j.bmcl.2015.12.016 BindingDB Entry DOI: 10.7270/Q2RV0QJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM10880 (AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard (Hamdard University) Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase-2 using p-nitrophenyl acetate as substrate by esterase assay | Bioorg Med Chem Lett 26: 1337-41 (2016) Article DOI: 10.1016/j.bmcl.2015.12.016 BindingDB Entry DOI: 10.7270/Q2RV0QJX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 5'-nucleotidase (Homo sapiens (Human)) | BDBM50165144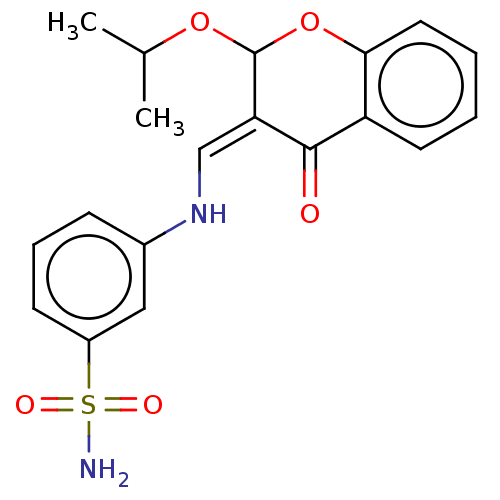 (CHEMBL3798753) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Forman Christian College (A Chartered University) Curated by ChEMBL | Assay Description Inhibition of human Ecto-5'-nucleotidase transfected in COS7 cells preincubated for 10 mins followed by AMP addition measured after 10 mins | Eur J Med Chem 115: 484-94 (2016) Article DOI: 10.1016/j.ejmech.2016.02.073 BindingDB Entry DOI: 10.7270/Q2K939D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM10890 (1-N-(3-chloro-1H-indol-7-yl)benzene-1,4-disulfonam...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard (Hamdard University) Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase-2 using p-nitrophenyl acetate as substrate by esterase assay | Bioorg Med Chem Lett 26: 1337-41 (2016) Article DOI: 10.1016/j.bmcl.2015.12.016 BindingDB Entry DOI: 10.7270/Q2RV0QJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Homo sapiens (Human)) | BDBM50165146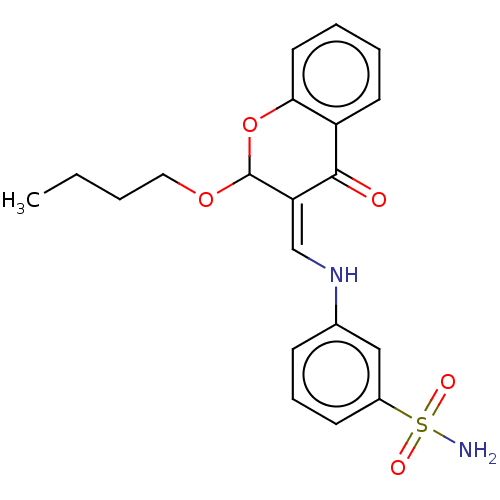 (CHEMBL3798795) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Forman Christian College (A Chartered University) Curated by ChEMBL | Assay Description Inhibition of human Ecto-5'-nucleotidase transfected in COS7 cells preincubated for 10 mins followed by AMP addition measured after 10 mins | Eur J Med Chem 115: 484-94 (2016) Article DOI: 10.1016/j.ejmech.2016.02.073 BindingDB Entry DOI: 10.7270/Q2K939D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Homo sapiens (Human)) | BDBM50165147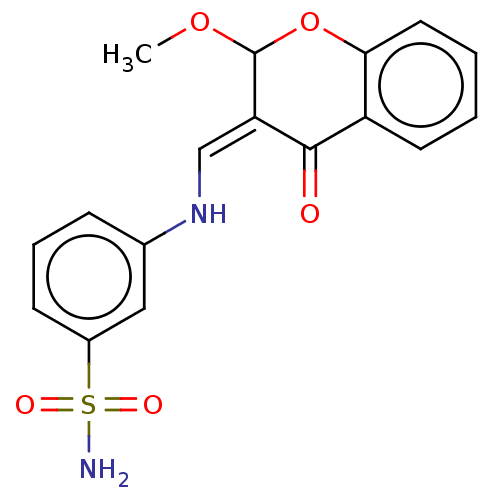 (CHEMBL3797302) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Forman Christian College (A Chartered University) Curated by ChEMBL | Assay Description Inhibition of human Ecto-5'-nucleotidase transfected in COS7 cells preincubated for 10 mins followed by AMP addition measured after 10 mins | Eur J Med Chem 115: 484-94 (2016) Article DOI: 10.1016/j.ejmech.2016.02.073 BindingDB Entry DOI: 10.7270/Q2K939D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Rattus norvegicus (Rat)) | BDBM50165143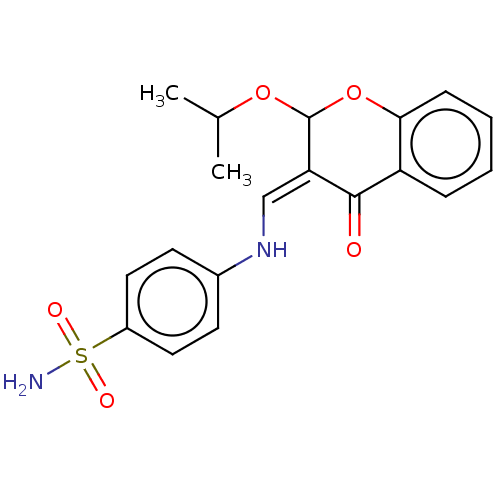 (CHEMBL3798555) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Forman Christian College (A Chartered University) Curated by ChEMBL | Assay Description Inhibition of rat Ecto-5'-nucleotidase transfected in COS7 cells preincubated for 10 mins followed by AMP addition measured after 10 mins | Eur J Med Chem 115: 484-94 (2016) Article DOI: 10.1016/j.ejmech.2016.02.073 BindingDB Entry DOI: 10.7270/Q2K939D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Homo sapiens (Human)) | BDBM50165149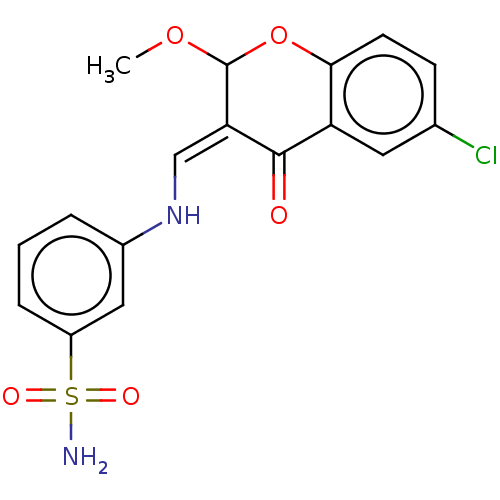 (CHEMBL3799691) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Forman Christian College (A Chartered University) Curated by ChEMBL | Assay Description Inhibition of human Ecto-5'-nucleotidase transfected in COS7 cells preincubated for 10 mins followed by AMP addition measured after 10 mins | Eur J Med Chem 115: 484-94 (2016) Article DOI: 10.1016/j.ejmech.2016.02.073 BindingDB Entry DOI: 10.7270/Q2K939D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Rattus norvegicus (Rat)) | BDBM50437939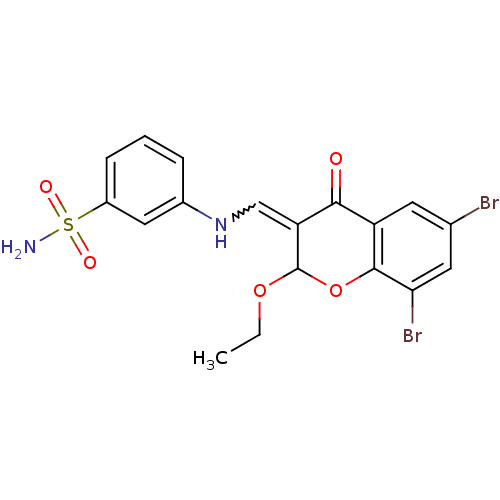 (CHEMBL1814399) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Forman Christian College (A Chartered University) Curated by ChEMBL | Assay Description Inhibition of rat Ecto-5'-nucleotidase transfected in COS7 cells preincubated for 10 mins followed by AMP addition measured after 10 mins | Eur J Med Chem 115: 484-94 (2016) Article DOI: 10.1016/j.ejmech.2016.02.073 BindingDB Entry DOI: 10.7270/Q2K939D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM10890 (1-N-(3-chloro-1H-indol-7-yl)benzene-1,4-disulfonam...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard (Hamdard University) Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase-1 using p-nitrophenyl acetate as substrate by esterase assay | Bioorg Med Chem Lett 26: 1337-41 (2016) Article DOI: 10.1016/j.bmcl.2015.12.016 BindingDB Entry DOI: 10.7270/Q2RV0QJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Homo sapiens (Human)) | BDBM50165137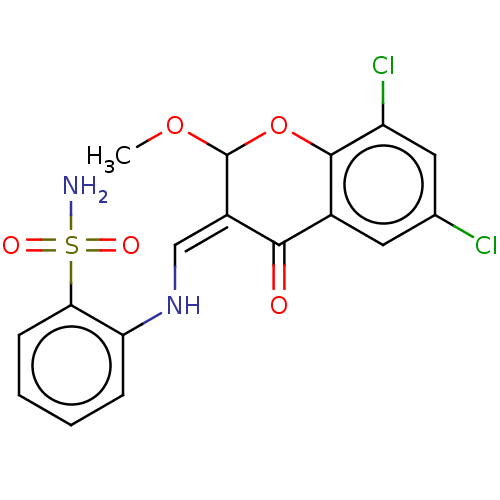 (CHEMBL3800324) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Forman Christian College (A Chartered University) Curated by ChEMBL | Assay Description Inhibition of human Ecto-5'-nucleotidase transfected in COS7 cells preincubated for 10 mins followed by AMP addition measured after 10 mins | Eur J Med Chem 115: 484-94 (2016) Article DOI: 10.1016/j.ejmech.2016.02.073 BindingDB Entry DOI: 10.7270/Q2K939D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Homo sapiens (Human)) | BDBM50165136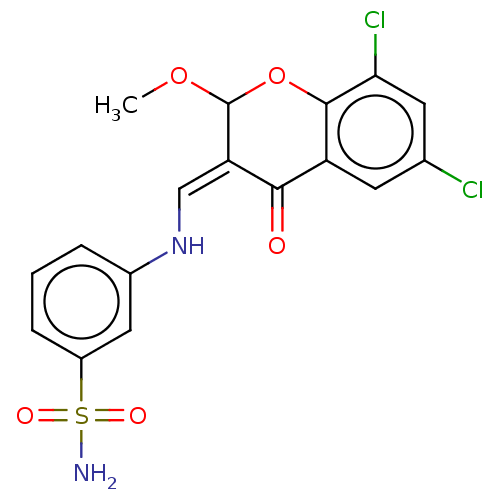 (CHEMBL3800429) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Forman Christian College (A Chartered University) Curated by ChEMBL | Assay Description Inhibition of human Ecto-5'-nucleotidase transfected in COS7 cells preincubated for 10 mins followed by AMP addition measured after 10 mins | Eur J Med Chem 115: 484-94 (2016) Article DOI: 10.1016/j.ejmech.2016.02.073 BindingDB Entry DOI: 10.7270/Q2K939D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Rattus norvegicus (Rat)) | BDBM50437941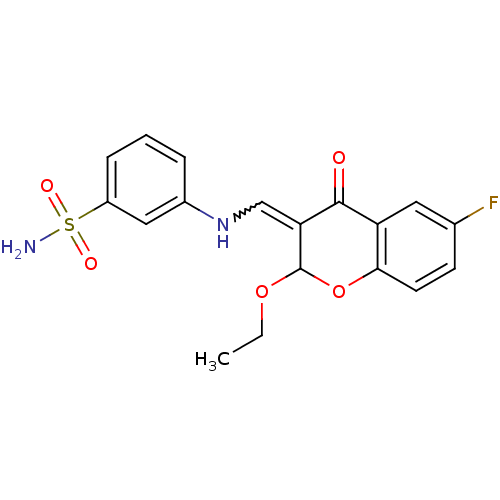 (CHEMBL1814397) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Forman Christian College (A Chartered University) Curated by ChEMBL | Assay Description Inhibition of rat Ecto-5'-nucleotidase transfected in COS7 cells preincubated for 10 mins followed by AMP addition measured after 10 mins | Eur J Med Chem 115: 484-94 (2016) Article DOI: 10.1016/j.ejmech.2016.02.073 BindingDB Entry DOI: 10.7270/Q2K939D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Rattus norvegicus (Rat)) | BDBM50165126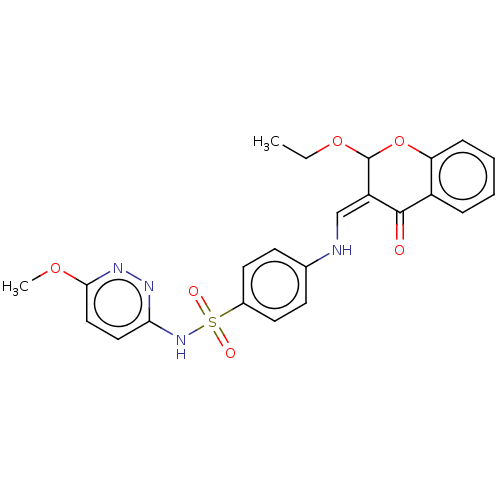 (CHEMBL3797319) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 52 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Forman Christian College (A Chartered University) Curated by ChEMBL | Assay Description Inhibition of rat Ecto-5'-nucleotidase transfected in COS7 cells preincubated for 10 mins followed by AMP addition measured after 10 mins | Eur J Med Chem 115: 484-94 (2016) Article DOI: 10.1016/j.ejmech.2016.02.073 BindingDB Entry DOI: 10.7270/Q2K939D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Homo sapiens (Human)) | BDBM50165143 (CHEMBL3798555) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 53 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Forman Christian College (A Chartered University) Curated by ChEMBL | Assay Description Inhibition of human Ecto-5'-nucleotidase transfected in COS7 cells preincubated for 10 mins followed by AMP addition measured after 10 mins | Eur J Med Chem 115: 484-94 (2016) Article DOI: 10.1016/j.ejmech.2016.02.073 BindingDB Entry DOI: 10.7270/Q2K939D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Rattus norvegicus (Rat)) | BDBM50165150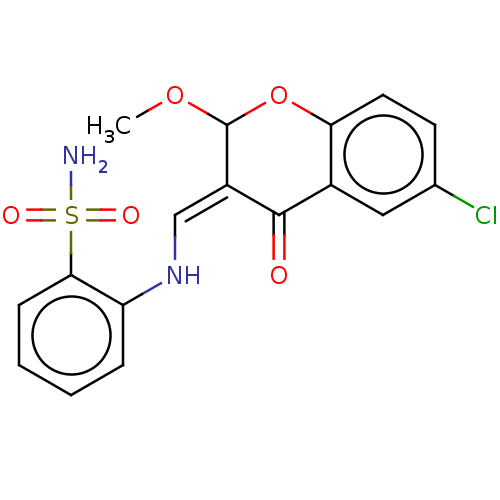 (CHEMBL3799027) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 62 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Forman Christian College (A Chartered University) Curated by ChEMBL | Assay Description Inhibition of rat Ecto-5'-nucleotidase transfected in COS7 cells preincubated for 10 mins followed by AMP addition measured after 10 mins | Eur J Med Chem 115: 484-94 (2016) Article DOI: 10.1016/j.ejmech.2016.02.073 BindingDB Entry DOI: 10.7270/Q2K939D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Homo sapiens (Human)) | BDBM50165145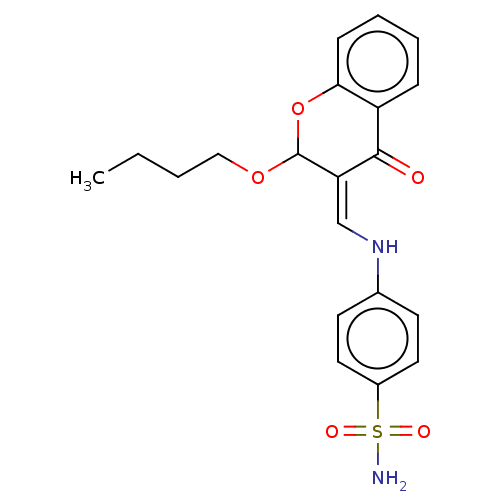 (CHEMBL3800455) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 63 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Forman Christian College (A Chartered University) Curated by ChEMBL | Assay Description Inhibition of human Ecto-5'-nucleotidase transfected in COS7 cells preincubated for 10 mins followed by AMP addition measured after 10 mins | Eur J Med Chem 115: 484-94 (2016) Article DOI: 10.1016/j.ejmech.2016.02.073 BindingDB Entry DOI: 10.7270/Q2K939D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Homo sapiens (Human)) | BDBM50165150 (CHEMBL3799027) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 64 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Forman Christian College (A Chartered University) Curated by ChEMBL | Assay Description Inhibition of human Ecto-5'-nucleotidase transfected in COS7 cells preincubated for 10 mins followed by AMP addition measured after 10 mins | Eur J Med Chem 115: 484-94 (2016) Article DOI: 10.1016/j.ejmech.2016.02.073 BindingDB Entry DOI: 10.7270/Q2K939D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alkaline phosphatase, tissue-nonspecific isozyme (Bos taurus (Cattle)) | BDBM50165140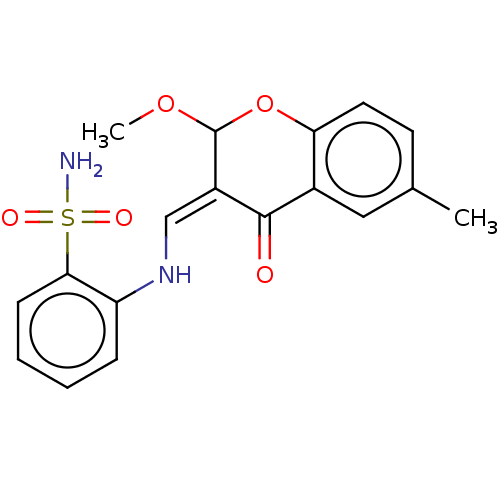 (CHEMBL3797297) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 78 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Forman Christian College (A Chartered University) Curated by ChEMBL | Assay Description Inhibition of bovine tissue non-specific alkaline phosphatase preincubated for 3 to 5 mins followed by CDP-star substrate addition measured after 15 ... | Eur J Med Chem 115: 484-94 (2016) Article DOI: 10.1016/j.ejmech.2016.02.073 BindingDB Entry DOI: 10.7270/Q2K939D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Homo sapiens (Human)) | BDBM50165148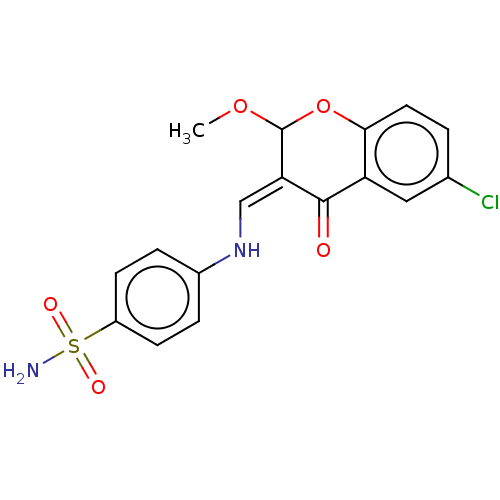 (CHEMBL3797640) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Forman Christian College (A Chartered University) Curated by ChEMBL | Assay Description Inhibition of human Ecto-5'-nucleotidase transfected in COS7 cells preincubated for 10 mins followed by AMP addition measured after 10 mins | Eur J Med Chem 115: 484-94 (2016) Article DOI: 10.1016/j.ejmech.2016.02.073 BindingDB Entry DOI: 10.7270/Q2K939D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Rattus norvegicus (Rat)) | BDBM50165148 (CHEMBL3797640) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 81 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Forman Christian College (A Chartered University) Curated by ChEMBL | Assay Description Inhibition of rat Ecto-5'-nucleotidase transfected in COS7 cells preincubated for 10 mins followed by AMP addition measured after 10 mins | Eur J Med Chem 115: 484-94 (2016) Article DOI: 10.1016/j.ejmech.2016.02.073 BindingDB Entry DOI: 10.7270/Q2K939D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Rattus norvegicus (Rat)) | BDBM50165137 (CHEMBL3800324) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 87 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Forman Christian College (A Chartered University) Curated by ChEMBL | Assay Description Inhibition of rat Ecto-5'-nucleotidase transfected in COS7 cells preincubated for 10 mins followed by AMP addition measured after 10 mins | Eur J Med Chem 115: 484-94 (2016) Article DOI: 10.1016/j.ejmech.2016.02.073 BindingDB Entry DOI: 10.7270/Q2K939D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Homo sapiens (Human)) | BDBM50165129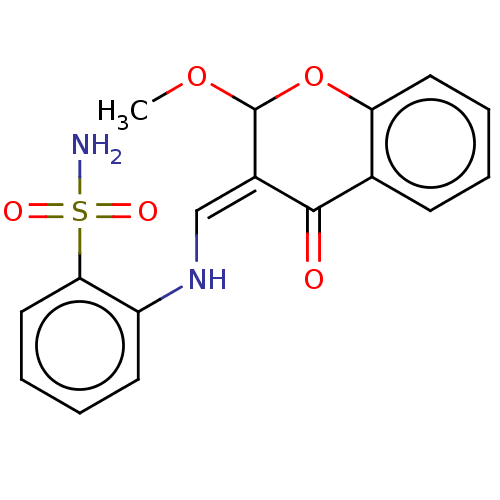 (CHEMBL3799469) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 91 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Forman Christian College (A Chartered University) Curated by ChEMBL | Assay Description Inhibition of human Ecto-5'-nucleotidase transfected in COS7 cells preincubated for 10 mins followed by AMP addition measured after 10 mins | Eur J Med Chem 115: 484-94 (2016) Article DOI: 10.1016/j.ejmech.2016.02.073 BindingDB Entry DOI: 10.7270/Q2K939D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Rattus norvegicus (Rat)) | BDBM50437946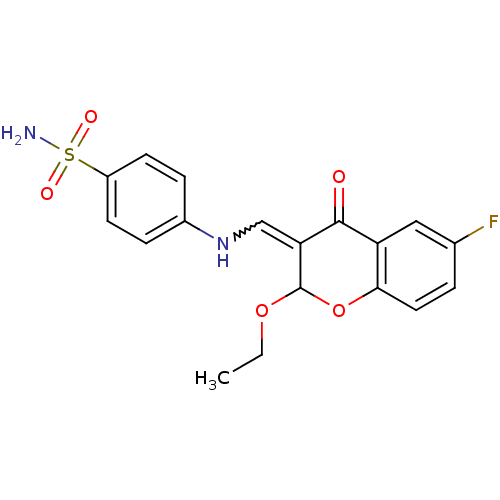 (CHEMBL1814392) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 101 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Forman Christian College (A Chartered University) Curated by ChEMBL | Assay Description Inhibition of rat Ecto-5'-nucleotidase transfected in COS7 cells preincubated for 10 mins followed by AMP addition measured after 10 mins | Eur J Med Chem 115: 484-94 (2016) Article DOI: 10.1016/j.ejmech.2016.02.073 BindingDB Entry DOI: 10.7270/Q2K939D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Rattus norvegicus (Rat)) | BDBM50165149 (CHEMBL3799691) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 113 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Forman Christian College (A Chartered University) Curated by ChEMBL | Assay Description Inhibition of rat Ecto-5'-nucleotidase transfected in COS7 cells preincubated for 10 mins followed by AMP addition measured after 10 mins | Eur J Med Chem 115: 484-94 (2016) Article DOI: 10.1016/j.ejmech.2016.02.073 BindingDB Entry DOI: 10.7270/Q2K939D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Rattus norvegicus (Rat)) | BDBM50165129 (CHEMBL3799469) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 114 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Forman Christian College (A Chartered University) Curated by ChEMBL | Assay Description Inhibition of rat Ecto-5'-nucleotidase transfected in COS7 cells preincubated for 10 mins followed by AMP addition measured after 10 mins | Eur J Med Chem 115: 484-94 (2016) Article DOI: 10.1016/j.ejmech.2016.02.073 BindingDB Entry DOI: 10.7270/Q2K939D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 448 total ) | Next | Last >> |