| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Acetylcholinesterase |
|---|
| Ligand | BDBM50187215 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_1589688 (CHEMBL3829843) |
|---|
| Kd | 6540±n/a nM |
|---|
| Citation |  Karade, HN; Raviraju, G; Acharya, BN; Valiveti, AK; Bhalerao, U; Acharya, J Synthesis and in vitro reactivation study of isonicotinamide derivatives of 2-(hydroxyimino)-N-(pyridin-3-yl)acetamide as reactivators of Sarin and VX inhibited human acetylcholinesterase (hAChE). Bioorg Med Chem24:4171-4176 (2016) [PubMed] Article Karade, HN; Raviraju, G; Acharya, BN; Valiveti, AK; Bhalerao, U; Acharya, J Synthesis and in vitro reactivation study of isonicotinamide derivatives of 2-(hydroxyimino)-N-(pyridin-3-yl)acetamide as reactivators of Sarin and VX inhibited human acetylcholinesterase (hAChE). Bioorg Med Chem24:4171-4176 (2016) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Acetylcholinesterase |
|---|
| Name: | Acetylcholinesterase |
|---|
| Synonyms: | ACES_HUMAN | ACHE | Acetylcholinesterase (AChE) | Acetylcholinesterase (human AChE) |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 67792.70 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P22303 |
|---|
| Residue: | 614 |
|---|
| Sequence: | MRPPQCLLHTPSLASPLLLLLLWLLGGGVGAEGREDAELLVTVRGGRLRGIRLKTPGGPV
SAFLGIPFAEPPMGPRRFLPPEPKQPWSGVVDATTFQSVCYQYVDTLYPGFEGTEMWNPN
RELSEDCLYLNVWTPYPRPTSPTPVLVWIYGGGFYSGASSLDVYDGRFLVQAERTVLVSM
NYRVGAFGFLALPGSREAPGNVGLLDQRLALQWVQENVAAFGGDPTSVTLFGESAGAASV
GMHLLSPPSRGLFHRAVLQSGAPNGPWATVGMGEARRRATQLAHLVGCPPGGTGGNDTEL
VACLRTRPAQVLVNHEWHVLPQESVFRFSFVPVVDGDFLSDTPEALINAGDFHGLQVLVG
VVKDEGSYFLVYGAPGFSKDNESLISRAEFLAGVRVGVPQVSDLAAEAVVLHYTDWLHPE
DPARLREALSDVVGDHNVVCPVAQLAGRLAAQGARVYAYVFEHRASTLSWPLWMGVPHGY
EIEFIFGIPLDPSRNYTAEEKIFAQRLMRYWANFARTGDPNEPRDPKAPQWPPYTAGAQQ
YVSLDLRPLEVRRGLRAQACAFWNRFLPKLLSATDTLDEAERQWKAEFHRWSSYMVHWKN
QFDHYSKQDRCSDL
|
|
|
|---|
| BDBM50187215 |
|---|
| n/a |
|---|
| Name | BDBM50187215 |
|---|
| Synonyms: | CHEMBL3828381 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C16H19Br2N5O3 |
|---|
| Mol. Mass. | 489.162 |
|---|
| SMILES | [Br-].[Br-].NC(=O)c1cc[n+](CCC[n+]2cccc(NC(=O)\C=N/O)c2)cc1 |
|---|
| Structure | 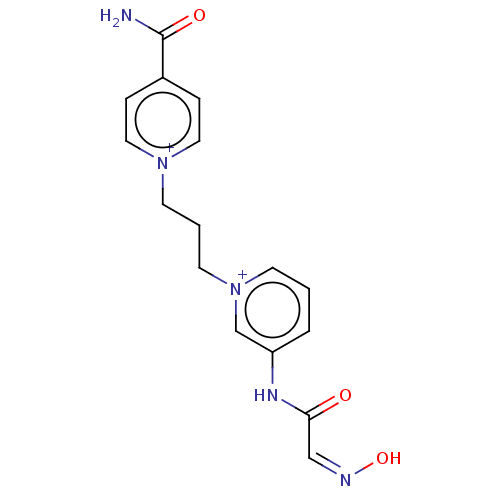
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Karade, HN; Raviraju, G; Acharya, BN; Valiveti, AK; Bhalerao, U; Acharya, J Synthesis and in vitro reactivation study of isonicotinamide derivatives of 2-(hydroxyimino)-N-(pyridin-3-yl)acetamide as reactivators of Sarin and VX inhibited human acetylcholinesterase (hAChE). Bioorg Med Chem24:4171-4176 (2016) [PubMed] Article
Karade, HN; Raviraju, G; Acharya, BN; Valiveti, AK; Bhalerao, U; Acharya, J Synthesis and in vitro reactivation study of isonicotinamide derivatives of 2-(hydroxyimino)-N-(pyridin-3-yl)acetamide as reactivators of Sarin and VX inhibited human acetylcholinesterase (hAChE). Bioorg Med Chem24:4171-4176 (2016) [PubMed] Article