| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Potassium voltage-gated channel subfamily H member 2 |
|---|
| Ligand | BDBM50031720 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_1617630 (CHEMBL3859699) |
|---|
| IC50 | 200±n/a nM |
|---|
| Citation |  Guo, D; Li, J; Lin, H; Zhou, Y; Chen, Y; Zhao, F; Sun, H; Zhang, D; Li, H; Shoichet, BK; Shan, L; Zhang, W; Xie, X; Jiang, H; Liu, H Design, Synthesis, and Biological Evaluation of Novel Tetrahydroprotoberberine Derivatives (THPBs) as Selectivea J Med Chem59:9489-9502 (2016) [PubMed] Article Guo, D; Li, J; Lin, H; Zhou, Y; Chen, Y; Zhao, F; Sun, H; Zhang, D; Li, H; Shoichet, BK; Shan, L; Zhang, W; Xie, X; Jiang, H; Liu, H Design, Synthesis, and Biological Evaluation of Novel Tetrahydroprotoberberine Derivatives (THPBs) as Selectivea J Med Chem59:9489-9502 (2016) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Potassium voltage-gated channel subfamily H member 2 |
|---|
| Name: | Potassium voltage-gated channel subfamily H member 2 |
|---|
| Synonyms: | 1,3-beta-glucan synthase component GLS2 | Cytochrome P450 3A4 | ERG | ERG1 | Eag-related protein 1 | Ether a-go-go related gene potassium channel (hERG) | Ether-a-go-go-related gene (HERG) | Ether-a-go-go-related gene potassium channel (hERG) | Ether-a-go-go-related gene potassium channel 1 | Ether-a-go-go-related gene potassium channel 1 (HERG) | Ether-a-go-go-related gene potassium channel 1 (hERG1) | Ether-a-go-go-related protein (hERG) | Ether-a-go-go-related protein 1 | Ether-a-go-go-related protein 1 (HERG) | H-ERG | HERG | KCNH2 | KCNH2_HUMAN | Potassium voltage-gated channel subfamily H member 2 (hERG) | Transcriptional regulator ERG | Voltage-gated potassium channel subunit Kv11.1 | eag homolog | hERG Potassium Channel 1 | putative potassium channel subunit |
|---|
| Type: | Multi-pass membrane protein |
|---|
| Mol. Mass.: | 126672.65 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | Q12809 |
|---|
| Residue: | 1159 |
|---|
| Sequence: | MPVRRGHVAPQNTFLDTIIRKFEGQSRKFIIANARVENCAVIYCNDGFCELCGYSRAEVM
QRPCTCDFLHGPRTQRRAAAQIAQALLGAEERKVEIAFYRKDGSCFLCLVDVVPVKNEDG
AVIMFILNFEVVMEKDMVGSPAHDTNHRGPPTSWLAPGRAKTFRLKLPALLALTARESSV
RSGGAGGAGAPGAVVVDVDLTPAAPSSESLALDEVTAMDNHVAGLGPAEERRALVGPGSP
PRSAPGQLPSPRAHSLNPDASGSSCSLARTRSRESCASVRRASSADDIEAMRAGVLPPPP
RHASTGAMHPLRSGLLNSTSDSDLVRYRTISKIPQITLNFVDLKGDPFLASPTSDREIIA
PKIKERTHNVTEKVTQVLSLGADVLPEYKLQAPRIHRWTILHYSPFKAVWDWLILLLVIY
TAVFTPYSAAFLLKETEEGPPATECGYACQPLAVVDLIVDIMFIVDILINFRTTYVNANE
EVVSHPGRIAVHYFKGWFLIDMVAAIPFDLLIFGSGSEELIGLLKTARLLRLVRVARKLD
RYSEYGAAVLFLLMCTFALIAHWLACIWYAIGNMEQPHMDSRIGWLHNLGDQIGKPYNSS
GLGGPSIKDKYVTALYFTFSSLTSVGFGNVSPNTNSEKIFSICVMLIGSLMYASIFGNVS
AIIQRLYSGTARYHTQMLRVREFIRFHQIPNPLRQRLEEYFQHAWSYTNGIDMNAVLKGF
PECLQADICLHLNRSLLQHCKPFRGATKGCLRALAMKFKTTHAPPGDTLVHAGDLLTALY
FISRGSIEILRGDVVVAILGKNDIFGEPLNLYARPGKSNGDVRALTYCDLHKIHRDDLLE
VLDMYPEFSDHFWSSLEITFNLRDTNMIPGSPGSTELEGGFSRQRKRKLSFRRRTDKDTE
QPGEVSALGPGRAGAGPSSRGRPGGPWGESPSSGPSSPESSEDEGPGRSSSPLRLVPFSS
PRPPGEPPGGEPLMEDCEKSSDTCNPLSGAFSGVSNIFSFWGDSRGRQYQELPRCPAPTP
SLLNIPLSSPGRRPRGDVESRLDALQRQLNRLETRLSADMATVLQLLQRQMTLVPPAYSA
VTTPGPGPTSTSPLLPVSPLPTLTLDSLSQVSQFMACEELPPGAPELPQEGPTRRLSLPG
QLGALTSQPLHRHGSDPGS
|
|
|
|---|
| BDBM50031720 |
|---|
| n/a |
|---|
| Name | BDBM50031720 |
|---|
| Synonyms: | (Dofetilide) N-[4-(2-{[2-(4-Methanesulfonylamino-phenyl)-ethyl]-methylamino}-ethoxy)-phenyl]-methanesulfonamide | CHEMBL473 | DOFETILIDE | N-[4-(2-{[2-(4-METHANESULFONYLAMINO-PHENYL)-ETHYL]-METHYL-AMINO}-ETHOXY)-PHENYL]-METHANESULFONAMIDE DOFETILIDE | N-[4-(2-{[2-(4-Methanesulfonylamino-phenyl)-ethyl]-methyl-amino}-ethoxy)-phenyl]-methanesulfonamide (UK-68798) | N-[4-(2-{[2-(4-Methanesulfonylamino-phenyl)-ethyl]-methyl-amino}-ethoxy)-phenyl]-methanesulfonamide (dofetilide) | N-[4-(2-{[2-(4-methanesulfonylamino-phenoxy)-ethyl]-methyl-amino}-ethyl)-phenyl]-methanesulfonamide | TIKOSYN | UK-68,798 | US10167299, Dofetilide |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C19H27N3O5S2 |
|---|
| Mol. Mass. | 441.565 |
|---|
| SMILES | CN(CCOc1ccc(NS(C)(=O)=O)cc1)CCc1ccc(NS(C)(=O)=O)cc1 |
|---|
| Structure | 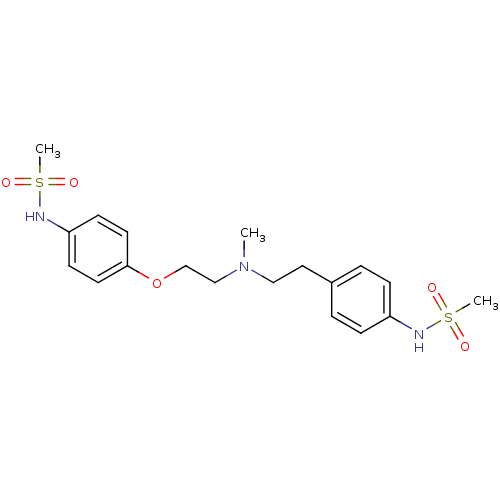
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Guo, D; Li, J; Lin, H; Zhou, Y; Chen, Y; Zhao, F; Sun, H; Zhang, D; Li, H; Shoichet, BK; Shan, L; Zhang, W; Xie, X; Jiang, H; Liu, H Design, Synthesis, and Biological Evaluation of Novel Tetrahydroprotoberberine Derivatives (THPBs) as Selectivea J Med Chem59:9489-9502 (2016) [PubMed] Article
Guo, D; Li, J; Lin, H; Zhou, Y; Chen, Y; Zhao, F; Sun, H; Zhang, D; Li, H; Shoichet, BK; Shan, L; Zhang, W; Xie, X; Jiang, H; Liu, H Design, Synthesis, and Biological Evaluation of Novel Tetrahydroprotoberberine Derivatives (THPBs) as Selectivea J Med Chem59:9489-9502 (2016) [PubMed] Article