| Synonyms: | PI3-kinase p110 subunit gamma | PI3-kinase subunit p120-gamma | PI3Kgamma | PIK3CG | PK3CG_HUMAN | Phosphatidylinositol 4,5-biphosphate 3-kinase catalytic subunit gamma (PIK3CG) | Phosphatidylinositol 4,5-bisphosphate 3-kinase (PI3K) | Phosphatidylinositol 4,5-bisphosphate 3-kinase 110 kDa catalytic subunit gamma (PI3K gamma) | Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma (PI3Kgamma) | Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform (PI3K gamma) | Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform (PI3K) | Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform (PI3Kgamma) | Phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit gamma isoform | Phosphoinositide 3-Kinase (PI3K), gamma Chain A | Phosphoinositide 3-kinases gamma (PI3K gamma) | Phosphoinositide-3-kinase (PI3K gamma) | p120-PI3K |
|---|
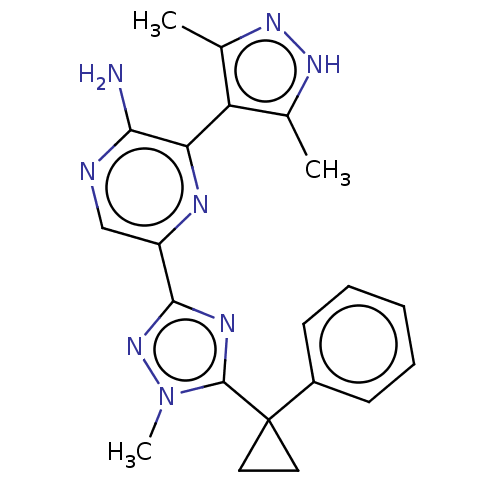
| SMILES | Cc1n[nH]c(C)c1-c1nc(cnc1N)-c1nc(n(C)n1)C1(CC1)c1ccccc1 |(25.94,-39.46,;25.61,-37.95,;26.63,-36.8,;25.86,-35.48,;24.36,-35.81,;23.2,-34.79,;24.21,-37.34,;22.88,-38.12,;21.55,-37.36,;20.22,-38.13,;20.22,-39.67,;21.55,-40.44,;22.89,-39.67,;24.22,-40.44,;18.88,-37.35,;18.72,-35.82,;17.22,-35.5,;16.45,-36.83,;14.92,-36.99,;17.48,-37.98,;16.54,-34.1,;15.21,-34.87,;15.21,-33.33,;17.29,-32.76,;18.83,-32.73,;19.57,-31.39,;18.78,-30.06,;17.23,-30.1,;16.49,-31.45,)| |
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Terstiege, I; Perry, M; Petersen, J; Tyrchan, C; Svensson, T; Lindmark, H; ึster, L Discovery of triazole aminopyrazines as a highly potent and selective series of PI3Kd inhibitors. Bioorg Med Chem Lett27:679-687 (2017) [PubMed] Article
Terstiege, I; Perry, M; Petersen, J; Tyrchan, C; Svensson, T; Lindmark, H; ึster, L Discovery of triazole aminopyrazines as a highly potent and selective series of PI3Kd inhibitors. Bioorg Med Chem Lett27:679-687 (2017) [PubMed] Article