| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Acetylcholinesterase |
|---|
| Ligand | BDBM152512 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | In Vitro AChE Inhibition Assay |
|---|
| pH | 8±n/a |
|---|
| Ki | 1.577e+4± 3e+1 nM |
|---|
| IC50 | 5.728e+4± 4e+1 nM |
|---|
| Km | 1160±2260 nM |
|---|
| Comments | extracted |
|---|
| Citation |  Piplani, P; Danta, CC Design and synthesis of newer potential 4-(N-acetylamino)phenol derived piperazine derivatives as potential cognition enhancers. Bioorg Chem60:64-73 (2015) [PubMed] Article Piplani, P; Danta, CC Design and synthesis of newer potential 4-(N-acetylamino)phenol derived piperazine derivatives as potential cognition enhancers. Bioorg Chem60:64-73 (2015) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Acetylcholinesterase |
|---|
| Name: | Acetylcholinesterase |
|---|
| Synonyms: | ACES_MOUSE | Acetylcholinesterase (AChE) | Acetylcholinesterase (mouse AChE) | Acetylcholinesterase precursor | Ache |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 68165.65 |
|---|
| Organism: | Mus musculus (mouse) |
|---|
| Description: | n/a |
|---|
| Residue: | 614 |
|---|
| Sequence: | MRPPWYPLHTPSLAFPLLFLLLSLLGGGARAEGREDPQLLVRVRGGQLRGIRLKAPGGPV
SAFLGIPFAEPPVGSRRFMPPEPKRPWSGVLDATTFQNVCYQYVDTLYPGFEGTEMWNPN
RELSEDCLYLNVWTPYPRPASPTPVLIWIYGGGFYSGAASLDVYDGRFLAQVEGAVLVSM
NYRVGTFGFLALPGSREAPGNVGLLDQRLALQWVQENIAAFGGDPMSVTLFGESAGAASV
GMHILSLPSRSLFHRAVLQSGTPNGPWATVSAGEARRRATLLARLVGCPPGGAGGNDTEL
IACLRTRPAQDLVDHEWHVLPQESIFRFSFVPVVDGDFLSDTPEALINTGDFQDLQVLVG
VVKDEGSYFLVYGVPGFSKDNESLISRAQFLAGVRIGVPQASDLAAEAVVLHYTDWLHPE
DPTHLRDAMSAVVGDHNVVCPVAQLAGRLAAQGARVYAYIFEHRASTLTWPLWMGVPHGY
EIEFIFGLPLDPSLNYTTEERIFAQRLMKYWTNFARTGDPNDPRDSKSPQWPPYTTAAQQ
YVSLNLKPLEVRRGLRAQTCAFWNRFLPKLLSATDTLDEAERQWKAEFHRWSSYMVHWKN
QFDHYSKQERCSDL
|
|
|
|---|
| BDBM152512 |
|---|
| n/a |
|---|
| Name | BDBM152512 |
|---|
| Synonyms: | N-[4-{2-(4-ethylpiperazin-1-yl)-2-oxoethoxy}phenyl]acetamide (1a) |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C16H23N3O3 |
|---|
| Mol. Mass. | 305.3721 |
|---|
| SMILES | CCN1CCN(CC1)C(=O)COc1ccc(NC(C)=O)cc1 |
|---|
| Structure | 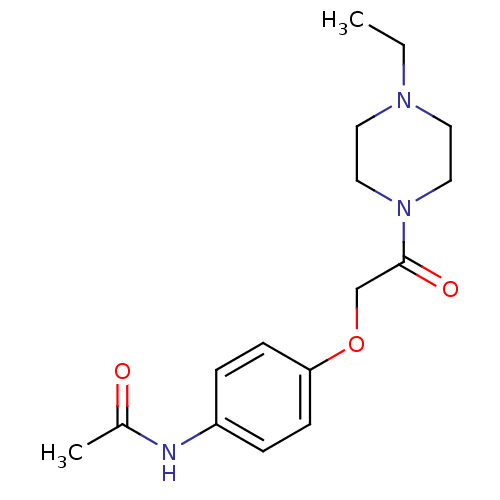
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Piplani, P; Danta, CC Design and synthesis of newer potential 4-(N-acetylamino)phenol derived piperazine derivatives as potential cognition enhancers. Bioorg Chem60:64-73 (2015) [PubMed] Article
Piplani, P; Danta, CC Design and synthesis of newer potential 4-(N-acetylamino)phenol derived piperazine derivatives as potential cognition enhancers. Bioorg Chem60:64-73 (2015) [PubMed] Article