| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Acetylcholinesterase |
|---|
| Ligand | BDBM152515 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | In Vitro AChE Inhibition Assay |
|---|
| pH | 8±n/a |
|---|
| Ki | 5.45e+3± 9.1e+2 nM |
|---|
| IC50 | 4.363e+4± 5e+1 nM |
|---|
| Km | 2160±3250 nM |
|---|
| Comments | extracted |
|---|
| Citation |  Piplani, P; Danta, CC Design and synthesis of newer potential 4-(N-acetylamino)phenol derived piperazine derivatives as potential cognition enhancers. Bioorg Chem60:64-73 (2015) [PubMed] Article Piplani, P; Danta, CC Design and synthesis of newer potential 4-(N-acetylamino)phenol derived piperazine derivatives as potential cognition enhancers. Bioorg Chem60:64-73 (2015) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Acetylcholinesterase |
|---|
| Name: | Acetylcholinesterase |
|---|
| Synonyms: | ACES_MOUSE | Acetylcholinesterase (AChE) | Acetylcholinesterase (mouse AChE) | Acetylcholinesterase precursor | Ache |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 68165.65 |
|---|
| Organism: | Mus musculus (mouse) |
|---|
| Description: | n/a |
|---|
| Residue: | 614 |
|---|
| Sequence: | MRPPWYPLHTPSLAFPLLFLLLSLLGGGARAEGREDPQLLVRVRGGQLRGIRLKAPGGPV
SAFLGIPFAEPPVGSRRFMPPEPKRPWSGVLDATTFQNVCYQYVDTLYPGFEGTEMWNPN
RELSEDCLYLNVWTPYPRPASPTPVLIWIYGGGFYSGAASLDVYDGRFLAQVEGAVLVSM
NYRVGTFGFLALPGSREAPGNVGLLDQRLALQWVQENIAAFGGDPMSVTLFGESAGAASV
GMHILSLPSRSLFHRAVLQSGTPNGPWATVSAGEARRRATLLARLVGCPPGGAGGNDTEL
IACLRTRPAQDLVDHEWHVLPQESIFRFSFVPVVDGDFLSDTPEALINTGDFQDLQVLVG
VVKDEGSYFLVYGVPGFSKDNESLISRAQFLAGVRIGVPQASDLAAEAVVLHYTDWLHPE
DPTHLRDAMSAVVGDHNVVCPVAQLAGRLAAQGARVYAYIFEHRASTLTWPLWMGVPHGY
EIEFIFGLPLDPSLNYTTEERIFAQRLMKYWTNFARTGDPNDPRDSKSPQWPPYTTAAQQ
YVSLNLKPLEVRRGLRAQTCAFWNRFLPKLLSATDTLDEAERQWKAEFHRWSSYMVHWKN
QFDHYSKQERCSDL
|
|
|
|---|
| BDBM152515 |
|---|
| n/a |
|---|
| Name | BDBM152515 |
|---|
| Synonyms: | N-(4-(2-(4-(4-fluorophenyl)piperazin-1-yl)-2-oxoethoxy)phenyl)acetamide (1d) |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C20H22FN3O3 |
|---|
| Mol. Mass. | 371.4054 |
|---|
| SMILES | CC(=O)Nc1ccc(OCC(=O)N2CCN(CC2)c2ccc(F)cc2)cc1 |
|---|
| Structure | 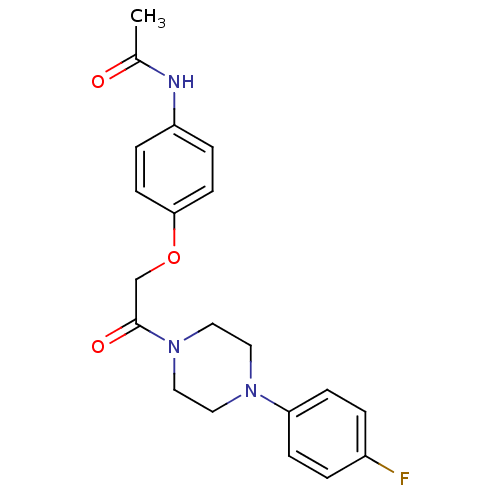
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Piplani, P; Danta, CC Design and synthesis of newer potential 4-(N-acetylamino)phenol derived piperazine derivatives as potential cognition enhancers. Bioorg Chem60:64-73 (2015) [PubMed] Article
Piplani, P; Danta, CC Design and synthesis of newer potential 4-(N-acetylamino)phenol derived piperazine derivatives as potential cognition enhancers. Bioorg Chem60:64-73 (2015) [PubMed] Article