| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Glycogen synthase kinase-3 beta |
|---|
| Ligand | BDBM189386 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | Kinase-Glo Assay |
|---|
| Temperature | 303.15±n/a K |
|---|
| IC50 | 3.1e+2± 3e+1 nM |
|---|
| Comments | extracted |
|---|
| Citation |  Tantray, MA; Khan, I; Hamid, H; Alam, MS; Umar, S; Ali, Y; Sharma, K; Hussain, F Synthesis of Novel Oxazolo[4,5-b]pyridine-2-one based 1,2,3-triazoles as Glycogen Synthase Kinase-3ß Inhibitors with Anti-inflammatory Potential. Chem Biol Drug Des87:918-26 (2016) [PubMed] Article Tantray, MA; Khan, I; Hamid, H; Alam, MS; Umar, S; Ali, Y; Sharma, K; Hussain, F Synthesis of Novel Oxazolo[4,5-b]pyridine-2-one based 1,2,3-triazoles as Glycogen Synthase Kinase-3ß Inhibitors with Anti-inflammatory Potential. Chem Biol Drug Des87:918-26 (2016) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Glycogen synthase kinase-3 beta |
|---|
| Name: | Glycogen synthase kinase-3 beta |
|---|
| Synonyms: | GSK-3 beta | GSK-3, beta | GSK3B | GSK3B_HUMAN | Glycogen synthase kinase 3 beta (GSK3B) | Glycogen synthase kinase 3-beta (GSK3B) | Glycogen synthase kinase-3 beta (GSK-3B) | Glycogen synthase kinase-3 beta (GSK3 Beta) | Glycogen synthase kinase-3 beta (GSK3B) | Glycogen synthase kinase-3B (GSK-3B) | Glycogen synthase kinase-3beta (GSK3B) | Serine/threonine-protein kinase GSK3B |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 46756.38 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P49841 |
|---|
| Residue: | 420 |
|---|
| Sequence: | MSGRPRTTSFAESCKPVQQPSAFGSMKVSRDKDGSKVTTVVATPGQGPDRPQEVSYTDTK
VIGNGSFGVVYQAKLCDSGELVAIKKVLQDKRFKNRELQIMRKLDHCNIVRLRYFFYSSG
EKKDEVYLNLVLDYVPETVYRVARHYSRAKQTLPVIYVKLYMYQLFRSLAYIHSFGICHR
DIKPQNLLLDPDTAVLKLCDFGSAKQLVRGEPNVSYICSRYYRAPELIFGATDYTSSIDV
WSAGCVLAELLLGQPIFPGDSGVDQLVEIIKVLGTPTREQIREMNPNYTEFKFPQIKAHP
WTKVFRPRTPPEAIALCSRLLEYTPTARLTPLEACAHSFFDELRDPNVKLPNGRDTPALF
NFTTQELSSNPPLATILIPPHARIQAAASTPTNATAASDANTGDRGQTNNAASASASNST
|
|
|
|---|
| BDBM189386 |
|---|
| n/a |
|---|
| Name | BDBM189386 |
|---|
| Synonyms: | 3-((1-(2-bromophenyl)-1H-1,2,3-triazol-4-yl)methyl)oxazolo[4,5-b]pyridin-2(3H)-one (4f) |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C15H10BrN5O2 |
|---|
| Mol. Mass. | 372.176 |
|---|
| SMILES | Brc1ccccc1-n1cc(Cn2c3ncccc3oc2=O)nn1 |
|---|
| Structure | 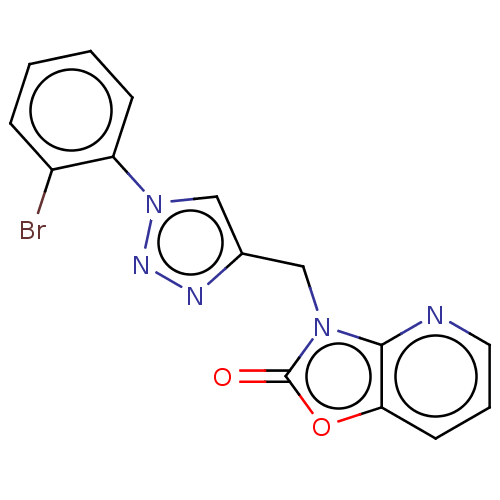
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Tantray, MA; Khan, I; Hamid, H; Alam, MS; Umar, S; Ali, Y; Sharma, K; Hussain, F Synthesis of Novel Oxazolo[4,5-b]pyridine-2-one based 1,2,3-triazoles as Glycogen Synthase Kinase-3ß Inhibitors with Anti-inflammatory Potential. Chem Biol Drug Des87:918-26 (2016) [PubMed] Article
Tantray, MA; Khan, I; Hamid, H; Alam, MS; Umar, S; Ali, Y; Sharma, K; Hussain, F Synthesis of Novel Oxazolo[4,5-b]pyridine-2-one based 1,2,3-triazoles as Glycogen Synthase Kinase-3ß Inhibitors with Anti-inflammatory Potential. Chem Biol Drug Des87:918-26 (2016) [PubMed] Article