

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Reaction Details | |||
|---|---|---|---|
 | Report a problem with these data | ||
| Target | Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform | ||
| Ligand | BDBM198095 | ||
| Substrate/Competitor | n/a | ||
| Meas. Tech. | Time-Resolved Fluorescence Resonance Energy transfer (TR-FRET) Assay | ||
| pH | 7.4±n/a | ||
| IC50 | 1±n/a nM | ||
| Comments | extracted | ||
| Citation |  Evarts, J; Kaplan, J; Kim, M; Patel, L; Perreault, S; Phillips, G; Treiberg, JA; Van Veldhuizen, J Phosphatidylinositol 3-kinase inhibitors US Patent US9221795 Publication Date 12/29/2015 Evarts, J; Kaplan, J; Kim, M; Patel, L; Perreault, S; Phillips, G; Treiberg, JA; Van Veldhuizen, J Phosphatidylinositol 3-kinase inhibitors US Patent US9221795 Publication Date 12/29/2015 | ||
| More Info.: | Get all data from this article, Assay Method | ||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform | |||
| Name: | Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform | ||
| Synonyms: | PI3-kinase p110 subunit beta | PI3-kinase subunit p110-beta | PI3Kbeta | PIK3C1 | PIK3CB | PK3CB_HUMAN | Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta (PI3Kbeta) | Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform (PI3K beta) | Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform (PI3K) | Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform (PI3K-beta) | Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform (PI3Kbeta) | Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform (PI3Kÿ²) | Phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit beta isoform | Phosphoinositide 3-Kinase (PI3K), beta | Phosphoinositide 3-Kinase (PI3K), beta Chain A | Phosphoinositide-3-kinase (PI3K beta) | PtdIns-3-kinase p110 | ||
| Type: | Enzyme Subunit | ||
| Mol. Mass.: | 122769.00 | ||
| Organism: | Homo sapiens (Human) | ||
| Description: | P42338 | ||
| Residue: | 1070 | ||
| Sequence: |
| ||
| BDBM198095 | |||
| n/a | |||
| Name | BDBM198095 | ||
| Synonyms: | BDBM198096 | US9221795, 91 | ||
| Type | Small organic molecule | ||
| Emp. Form. | C22H21ClN10O | ||
| Mol. Mass. | 476.922 | ||
| SMILES | CC[C@H](Nc1nc(N)nc(N)c1C#N)c1nc2cccc(Cl)c2c(=O)n1-c1cnc(N)cc1C |r,wD:2.2,(3.33,.38,;2,1.15,;.67,.38,;.67,-1.16,;2,-1.93,;3.33,-1.16,;4.67,-1.93,;6,-1.15,;4.67,-3.47,;3.33,-4.23,;3.33,-5.78,;2,-3.47,;.67,-4.24,;-.67,-5.01,;-.67,1.15,;-2,.38,;-3.33,1.15,;-4.67,.38,;-6,1.15,;-6,2.69,;-4.67,3.46,;-4.67,5,;-3.33,2.69,;-2,3.46,;-2,5,;-.67,2.69,;.67,3.46,;2,2.69,;3.33,3.46,;3.33,5,;4.67,5.77,;2,5.78,;.67,5,;-.67,5.77,)| | ||
| Structure | 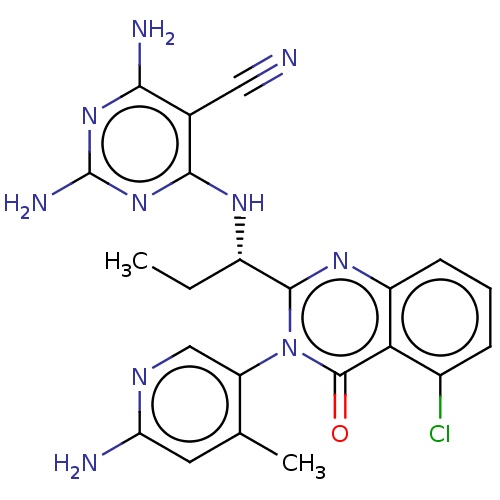
| ||