

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coagulation factor X (Homo sapiens (Human)) | BDBM17284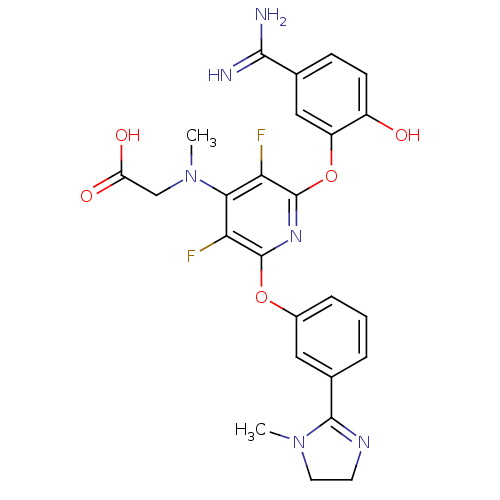 (2-{[2-(5-carbamimidoyl-2-hydroxyphenoxy)-3,5-diflu...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences Curated by ChEMBL | Assay Description Inhibitory potency was measured against human coagulation factor X | J Med Chem 41: 3557-62 (1998) Article DOI: 10.1021/jm980280h BindingDB Entry DOI: 10.7270/Q2KS6QPR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM17280 (1-[2-(5-carbamimidoyl-2-hydroxyphenoxy)-3,5-difluo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.110 | -56.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Berlex | Assay Description The enzyme activities were determined kinetically as the initial rate of cleavage of a peptide p-nitroanilide. Km for enzyme and substrate was determ... | Biochemistry 39: 12534-42 (2000) Article DOI: 10.1021/bi001477q BindingDB Entry DOI: 10.7270/Q2C827JM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM17284 (2-{[2-(5-carbamimidoyl-2-hydroxyphenoxy)-3,5-diflu...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 0.110 | -56.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Berlex | Assay Description The enzyme activities were determined kinetically as the initial rate of cleavage of a peptide p-nitroanilide. Km for enzyme and substrate was determ... | Biochemistry 39: 12534-42 (2000) Article DOI: 10.1021/bi001477q BindingDB Entry DOI: 10.7270/Q2C827JM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM17280 (1-[2-(5-carbamimidoyl-2-hydroxyphenoxy)-3,5-difluo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.110 | -56.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Berlex | Assay Description The enzyme activities were determined kinetically as the initial rate of cleavage of a peptide p-nitroanilide. Km for enzyme and substrate was determ... | Acta Crystallogr D Biol Crystallogr 55: 1395-404 (1999) Article DOI: 10.1107/s0907444999007350 BindingDB Entry DOI: 10.7270/Q2H1308B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50074629 (4-[(1R,2R)-2-(5,5-Dimethyl-hex-1-ynyl)-cyclopropyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gliatech Inc. Curated by ChEMBL | Assay Description Binding affinity at histamine H3 receptor in rat cortical membranes by [3H]-Nalpha-methylhistamine displacement. | J Med Chem 42: 903-9 (1999) Article DOI: 10.1021/jm980310g BindingDB Entry DOI: 10.7270/Q2ST7P1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM22541 (Clobenpropit | N''-[(4-chlorophenyl)methyl]{[3-(1H...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Histamine H3 receptor affinity of compound was determined in rat cortical membranes using the H3 selective agonist ligand, [3H]N-alpha-methylhistamin... | Bioorg Med Chem Lett 7: 3017-3022 (1997) Article DOI: 10.1016/S0960-894X(97)10137-8 BindingDB Entry DOI: 10.7270/Q2280843 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM22541 (Clobenpropit | N''-[(4-chlorophenyl)methyl]{[3-(1H...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gliatech Inc. Curated by ChEMBL | Assay Description Inhibition of [3H]- N-alpha-methylhistamine from histamine H3 receptor | Bioorg Med Chem Lett 8: 1133-8 (1999) BindingDB Entry DOI: 10.7270/Q2GF0SPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50066635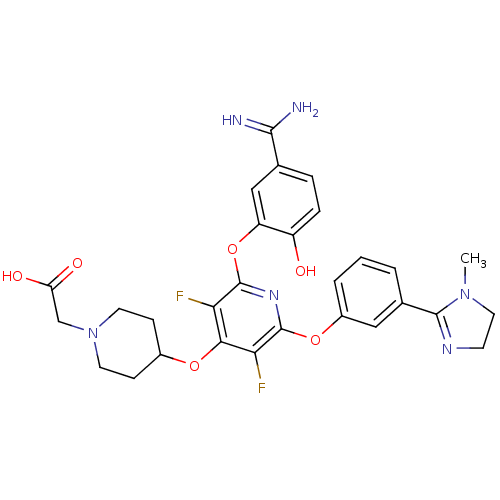 ((4-{2-(5-Carbamimidoyl-2-hydroxy-phenoxy)-3,5-difl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences Curated by ChEMBL | Assay Description Inhibitory potency was measured against human coagulation factor X | J Med Chem 41: 3557-62 (1998) Article DOI: 10.1021/jm980280h BindingDB Entry DOI: 10.7270/Q2KS6QPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50066619 (({2-(5-Carbamimidoyl-2-hydroxy-phenoxy)-3,5-difluo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences Curated by ChEMBL | Assay Description Inhibitory potency was measured against human coagulation factor X | J Med Chem 41: 3557-62 (1998) Article DOI: 10.1021/jm980280h BindingDB Entry DOI: 10.7270/Q2KS6QPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM17282 (7-({6-[(1-ethanimidoylpiperidin-4-yl)oxy]-2-methyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 0.270 | -54.1 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Berlex | Assay Description The enzyme activities were determined kinetically as the initial rate of cleavage of a peptide p-nitroanilide. Km for enzyme and substrate was determ... | Acta Crystallogr D Biol Crystallogr 55: 1395-404 (1999) Article DOI: 10.1107/s0907444999007350 BindingDB Entry DOI: 10.7270/Q2H1308B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM22916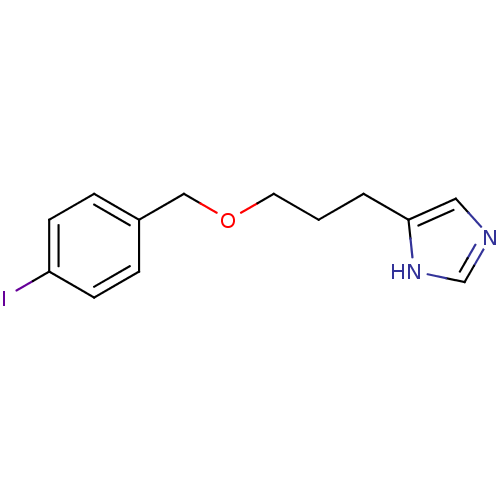 (5-{3-[(4-iodophenyl)methoxy]propyl}-1H-imidazole |...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Histamine H3 receptor affinity of compound was determined in rat cortical membranes using the H3 selective agonist ligand, [3H]N-alpha-methylhistamin... | Bioorg Med Chem Lett 7: 3017-3022 (1997) Article DOI: 10.1016/S0960-894X(97)10137-8 BindingDB Entry DOI: 10.7270/Q2280843 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM17259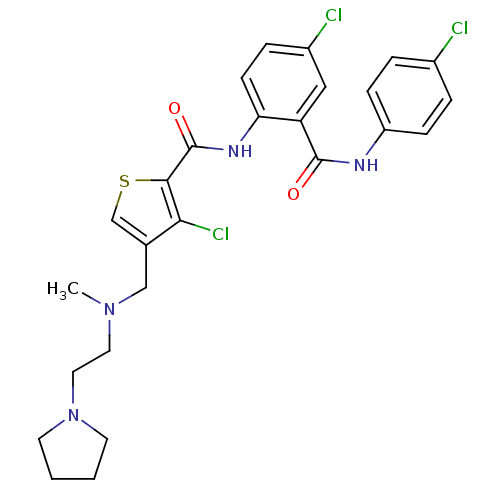 (3-chloro-N-{4-chloro-2-[(4-chlorophenyl)carbamoyl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex | Assay Description The enzyme activities were determined kinetically as the initial rate of cleavage of a peptide p-nitroanilide. Km for enzyme and substrate was determ... | Bioorg Med Chem 15: 2127-46 (2007) Article DOI: 10.1016/j.bmc.2006.12.019 BindingDB Entry DOI: 10.7270/Q2MS3R1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50066634 ((4-{2-(5-Carbamimidoyl-2-hydroxy-phenoxy)-3,5-difl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences Curated by ChEMBL | Assay Description Inhibitory potency was measured against human coagulation factor X | J Med Chem 41: 3557-62 (1998) Article DOI: 10.1021/jm980280h BindingDB Entry DOI: 10.7270/Q2KS6QPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM17226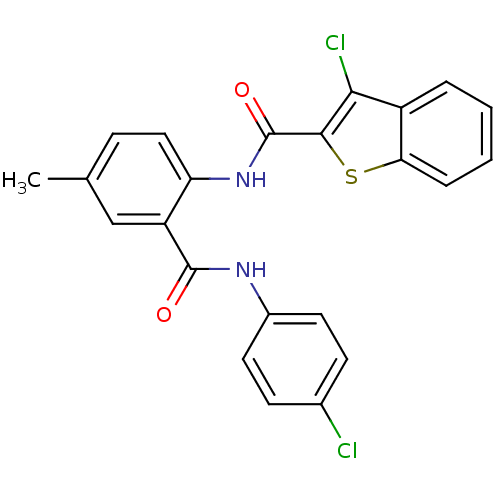 (3-chloro-N-{2-[(4-chlorophenyl)carbamoyl]-4-methyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | PubMed | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences Curated by ChEMBL | Assay Description Inhibition constant against human factor Xa | Bioorg Med Chem Lett 13: 507-11 (2003) BindingDB Entry DOI: 10.7270/Q21N829C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50066641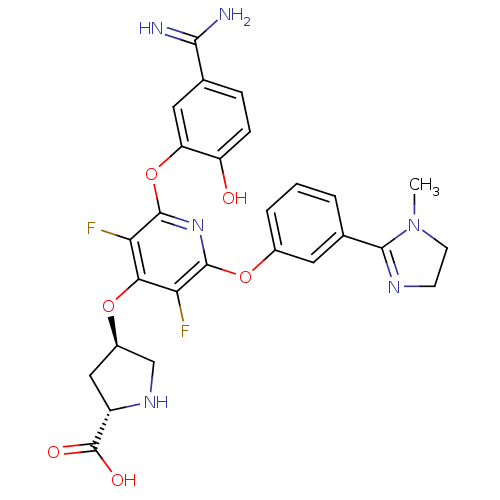 ((2S,4R)-4-{2-(5-Carbamimidoyl-2-hydroxy-phenoxy)-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences Curated by ChEMBL | Assay Description Inhibitory potency was measured against human coagulation factor X | J Med Chem 41: 3557-62 (1998) Article DOI: 10.1021/jm980280h BindingDB Entry DOI: 10.7270/Q2KS6QPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50074627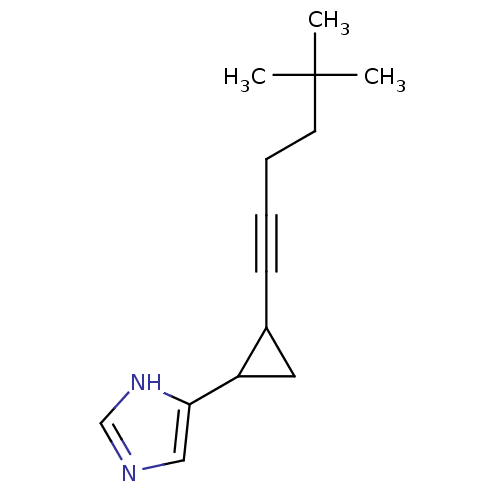 (4-[2-(5,5-Dimethyl-hex-1-ynyl)-cyclopropyl]-1H-imi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gliatech Inc. Curated by ChEMBL | Assay Description Binding affinity at histamine H3 receptor in rat cortical membranes by [3H]-Nalpha-methylhistamine displacement. | J Med Chem 42: 903-9 (1999) Article DOI: 10.1021/jm980310g BindingDB Entry DOI: 10.7270/Q2ST7P1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM17226 (3-chloro-N-{2-[(4-chlorophenyl)carbamoyl]-4-methyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.330 | -53.6 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Berlex | Assay Description The enzyme activities were determined kinetically as the initial rate of cleavage of a peptide p-nitroanilide. Km for enzyme and substrate was determ... | Bioorg Med Chem 15: 2127-46 (2007) Article DOI: 10.1016/j.bmc.2006.12.019 BindingDB Entry DOI: 10.7270/Q2MS3R1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM17261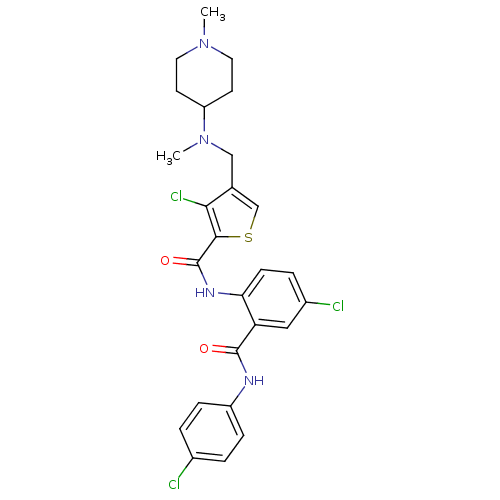 (3-chloro-N-{4-chloro-2-[(4-chlorophenyl)carbamoyl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex | Assay Description The enzyme activities were determined kinetically as the initial rate of cleavage of a peptide p-nitroanilide. Km for enzyme and substrate was determ... | Bioorg Med Chem 15: 2127-46 (2007) Article DOI: 10.1016/j.bmc.2006.12.019 BindingDB Entry DOI: 10.7270/Q2MS3R1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM17269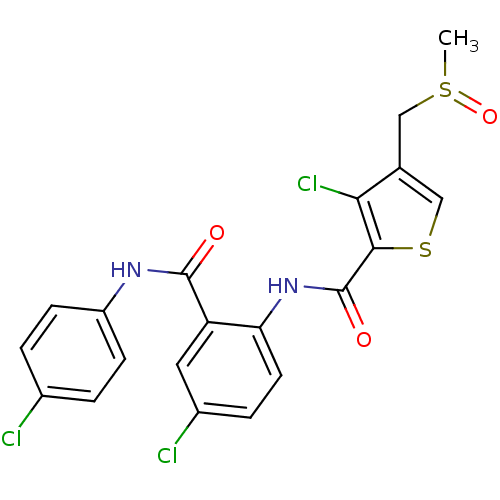 (3-chloro-N-{4-chloro-2-[(4-chlorophenyl)carbamoyl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex | Assay Description The enzyme activities were determined kinetically as the initial rate of cleavage of a peptide p-nitroanilide. Km for enzyme and substrate was determ... | Bioorg Med Chem 15: 2127-46 (2007) Article DOI: 10.1016/j.bmc.2006.12.019 BindingDB Entry DOI: 10.7270/Q2MS3R1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50066623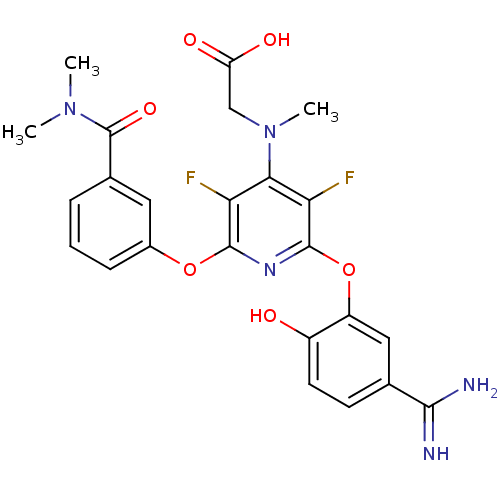 (CHEMBL72318 | {[2-(5-Carbamimidoyl-2-hydroxy-pheno...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences Curated by ChEMBL | Assay Description Inhibitory potency was measured against human coagulation factor X | J Med Chem 41: 3557-62 (1998) Article DOI: 10.1021/jm980280h BindingDB Entry DOI: 10.7270/Q2KS6QPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50066639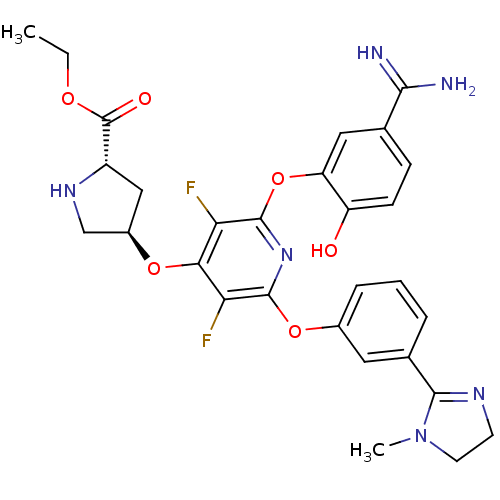 ((2S,4R)-4-{2-(5-Carbamimidoyl-2-hydroxy-phenoxy)-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences Curated by ChEMBL | Assay Description Inhibitory potency was measured against human coagulation factor X | J Med Chem 41: 3557-62 (1998) Article DOI: 10.1021/jm980280h BindingDB Entry DOI: 10.7270/Q2KS6QPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM17256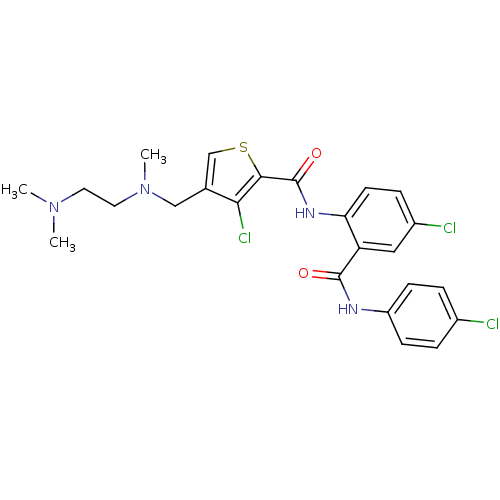 (3-chloro-N-{4-chloro-2-[(4-chlorophenyl)carbamoyl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex | Assay Description The enzyme activities were determined kinetically as the initial rate of cleavage of a peptide p-nitroanilide. Km for enzyme and substrate was determ... | Bioorg Med Chem 15: 2127-46 (2007) Article DOI: 10.1016/j.bmc.2006.12.019 BindingDB Entry DOI: 10.7270/Q2MS3R1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50404108 (CHEMBL147645) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences Curated by ChEMBL | Assay Description Inhibition constant against human factor Xa | Bioorg Med Chem Lett 13: 507-11 (2003) BindingDB Entry DOI: 10.7270/Q21N829C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50070217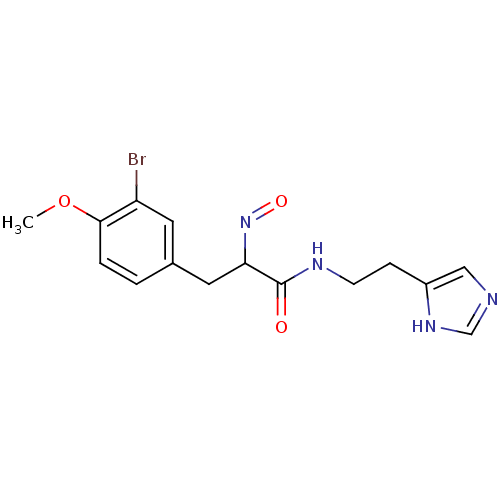 (3-(3-Bromo-4-methoxy-phenyl)-2-[(E)-hydroxyimino]-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gliatech Inc. Curated by ChEMBL | Assay Description Inhibition of [3H]- N-alpha-methylhistamine from histamine H3 receptor | Bioorg Med Chem Lett 8: 1133-8 (1999) BindingDB Entry DOI: 10.7270/Q2GF0SPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM21393 (7-(dipropylamino)-5,6,7,8-tetrahydronaphthalen-1-o...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Laboratories Curated by ChEMBL | Assay Description Binding affinity against [3H]- -8-OH-DPAT -labeled 5-hydroxytryptamine 1A sites in cloned CHO cells | J Med Chem 36: 2208-18 (1993) BindingDB Entry DOI: 10.7270/Q27D2T69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM17257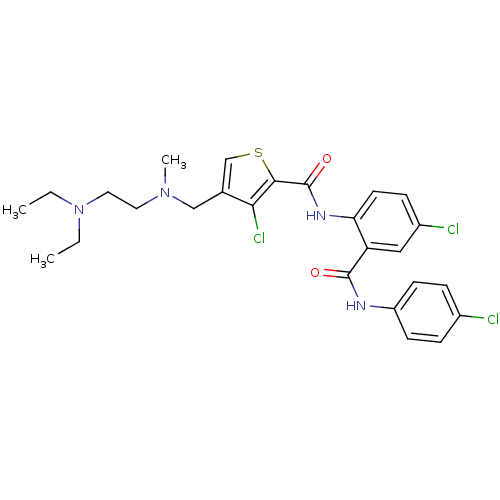 (3-chloro-N-{4-chloro-2-[(4-chlorophenyl)carbamoyl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex | Assay Description The enzyme activities were determined kinetically as the initial rate of cleavage of a peptide p-nitroanilide. Km for enzyme and substrate was determ... | Bioorg Med Chem 15: 2127-46 (2007) Article DOI: 10.1016/j.bmc.2006.12.019 BindingDB Entry DOI: 10.7270/Q2MS3R1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM17227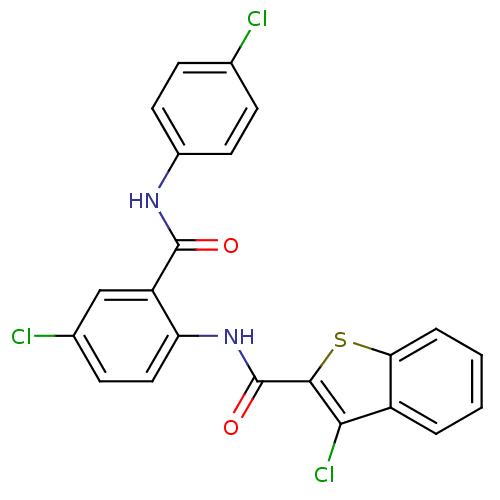 (3-chloro-N-{4-chloro-2-[(4-chlorophenyl)carbamoyl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.600 | -52.1 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Berlex | Assay Description The enzyme activities were determined kinetically as the initial rate of cleavage of a peptide p-nitroanilide. Km for enzyme and substrate was determ... | Bioorg Med Chem 15: 2127-46 (2007) Article DOI: 10.1016/j.bmc.2006.12.019 BindingDB Entry DOI: 10.7270/Q2MS3R1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Cavia porcellus) | BDBM50038757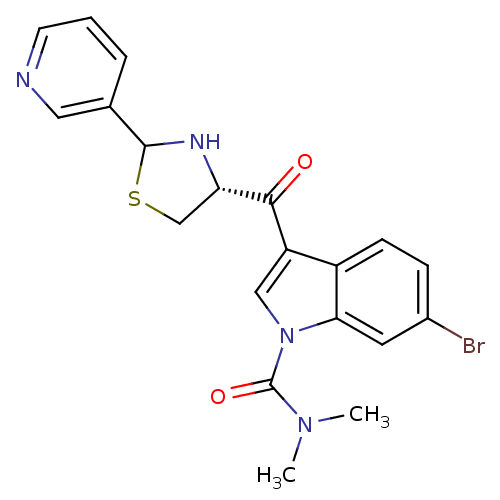 (6-Bromo-3-((R)-2-pyridin-3-yl-thiazolidine-4-carbo...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Antagonist activity against platelet activating factor (PAF) receptor in rabbit platelet membranes using [3H]C18-PAF as radioligand | J Med Chem 37: 2011-32 (1994) BindingDB Entry DOI: 10.7270/Q2GH9JM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM17258 (3-chloro-N-{4-chloro-2-[(4-chlorophenyl)carbamoyl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex | Assay Description The enzyme activities were determined kinetically as the initial rate of cleavage of a peptide p-nitroanilide. Km for enzyme and substrate was determ... | Bioorg Med Chem 15: 2127-46 (2007) Article DOI: 10.1016/j.bmc.2006.12.019 BindingDB Entry DOI: 10.7270/Q2MS3R1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM17227 (3-chloro-N-{4-chloro-2-[(4-chlorophenyl)carbamoyl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences Curated by ChEMBL | Assay Description Inhibition constant against human factor Xa | Bioorg Med Chem Lett 13: 507-11 (2003) BindingDB Entry DOI: 10.7270/Q21N829C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50066625 (CHEMBL120438 | {4-[2-(5-Carbamimidoyl-2-hydroxy-ph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences Curated by ChEMBL | Assay Description Inhibitory potency was measured against human coagulation factor X | J Med Chem 41: 3557-62 (1998) Article DOI: 10.1021/jm980280h BindingDB Entry DOI: 10.7270/Q2KS6QPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50066614 ((E,E)-2,7-Bis(4-amidinobenzylidine)cycloheptan-1-o...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences Curated by ChEMBL | Assay Description Compound was tested for inhibition activity against Coagulation factor X | J Med Chem 42: 1749-56 (1999) Article DOI: 10.1021/jm980667k BindingDB Entry DOI: 10.7270/Q2FX78NF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM17277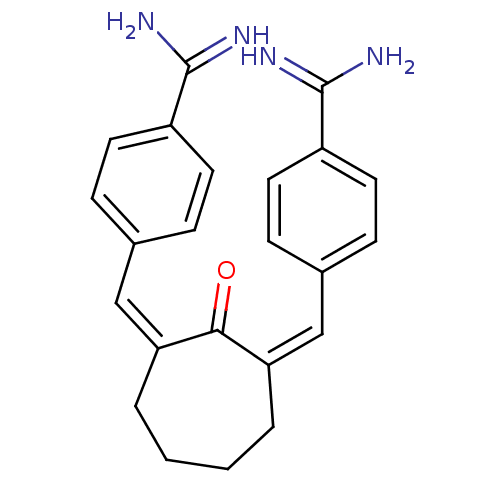 ((Z,Z)BABCH | 4-{[(1Z,3Z)-3-[(4-carbamimidoylphenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences Curated by ChEMBL | Assay Description Inhibitory potency was measured against human coagulation factor X | J Med Chem 41: 3557-62 (1998) Article DOI: 10.1021/jm980280h BindingDB Entry DOI: 10.7270/Q2KS6QPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM17277 ((Z,Z)BABCH | 4-{[(1Z,3Z)-3-[(4-carbamimidoylphenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.660 | -51.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Berlex | Assay Description The enzyme activities were determined kinetically as the initial rate of cleavage of a peptide p-nitroanilide. Km for enzyme and substrate was determ... | Acta Crystallogr D Biol Crystallogr 55: 1395-404 (1999) Article DOI: 10.1107/s0907444999007350 BindingDB Entry DOI: 10.7270/Q2H1308B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50066628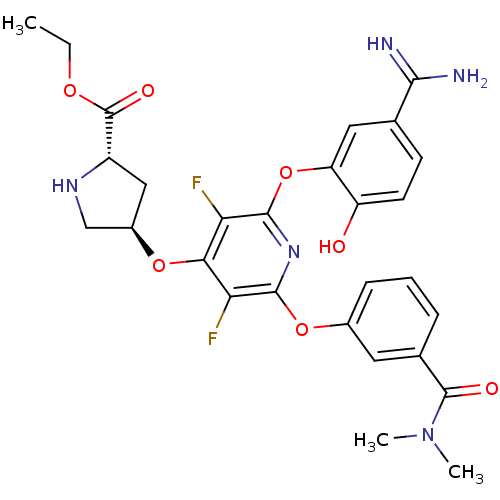 ((2S,4R)-4-[2-(5-Carbamimidoyl-2-hydroxy-phenoxy)-6...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences Curated by ChEMBL | Assay Description Inhibitory potency was measured against human coagulation factor X | J Med Chem 41: 3557-62 (1998) Article DOI: 10.1021/jm980280h BindingDB Entry DOI: 10.7270/Q2KS6QPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM17252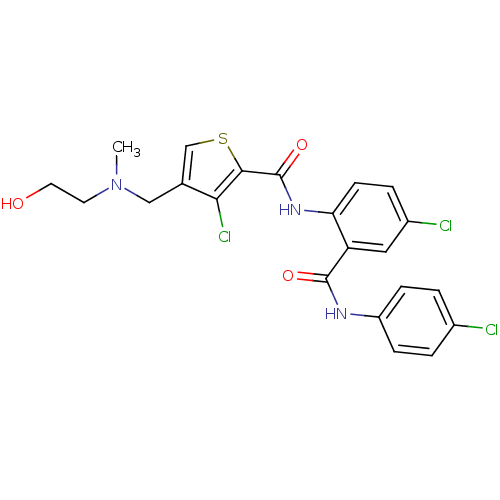 (3-chloro-N-{4-chloro-2-[(4-chlorophenyl)carbamoyl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex | Assay Description The enzyme activities were determined kinetically as the initial rate of cleavage of a peptide p-nitroanilide. Km for enzyme and substrate was determ... | Bioorg Med Chem 15: 2127-46 (2007) Article DOI: 10.1016/j.bmc.2006.12.019 BindingDB Entry DOI: 10.7270/Q2MS3R1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM17268 (3-chloro-N-{4-chloro-2-[(4-chlorophenyl)carbamoyl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex | Assay Description The enzyme activities were determined kinetically as the initial rate of cleavage of a peptide p-nitroanilide. Km for enzyme and substrate was determ... | Bioorg Med Chem 15: 2127-46 (2007) Article DOI: 10.1016/j.bmc.2006.12.019 BindingDB Entry DOI: 10.7270/Q2MS3R1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Cavia porcellus) | BDBM50038809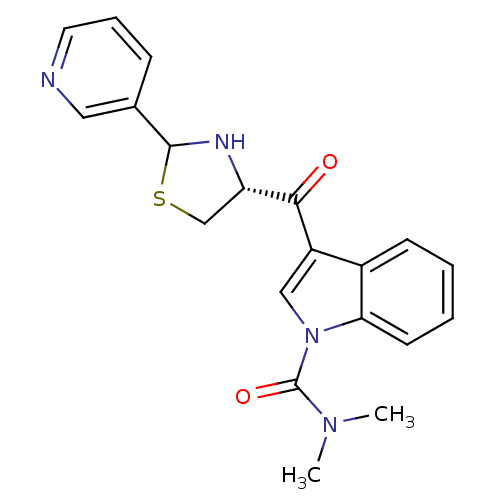 (3-((R)-2-Pyridin-3-yl-thiazolidine-4-carbonyl)-ind...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Antagonist activity against platelet activating factor (PAF) receptor in rabbit platelet membranes using [3H]C18-PAF as radioligand | J Med Chem 37: 2011-32 (1994) BindingDB Entry DOI: 10.7270/Q2GH9JM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50070214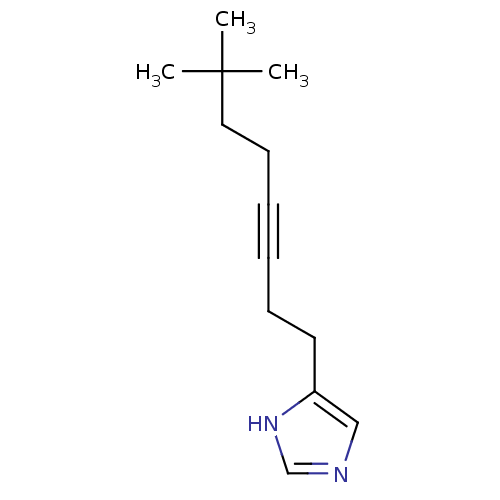 (4-(7,7-Dimethyl-oct-3-ynyl)-1H-imidazole | CHEMBL2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gliatech Inc. Curated by ChEMBL | Assay Description Binding affinity at histamine H3 receptor in rat cortical membranes by [3H]-Nalpha-methylhistamine displacement. | J Med Chem 42: 903-9 (1999) Article DOI: 10.1021/jm980310g BindingDB Entry DOI: 10.7270/Q2ST7P1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50070214 (4-(7,7-Dimethyl-oct-3-ynyl)-1H-imidazole | CHEMBL2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gliatech Inc. Curated by ChEMBL | Assay Description Inhibition of [3H]- N-alpha-methylhistamine from histamine H3 receptor | Bioorg Med Chem Lett 8: 1133-8 (1999) BindingDB Entry DOI: 10.7270/Q2GF0SPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM17228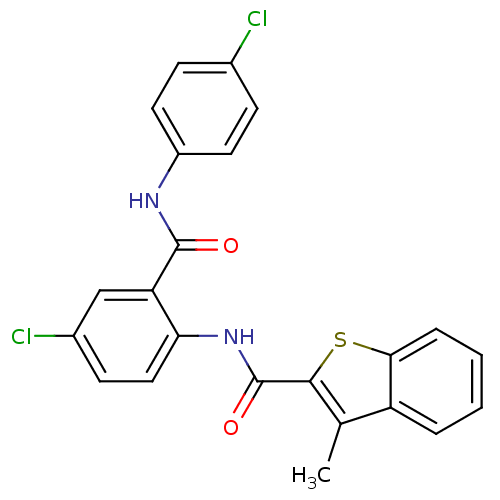 (N-{4-chloro-2-[(4-chlorophenyl)carbamoyl]phenyl}-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.820 | -51.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Berlex | Assay Description The enzyme activities were determined kinetically as the initial rate of cleavage of a peptide p-nitroanilide. Km for enzyme and substrate was determ... | Bioorg Med Chem 15: 2127-46 (2007) Article DOI: 10.1016/j.bmc.2006.12.019 BindingDB Entry DOI: 10.7270/Q2MS3R1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hrh3 protein (RAT) | BDBM50070214 (4-(7,7-Dimethyl-oct-3-ynyl)-1H-imidazole | CHEMBL2...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.830 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gliatech Inc. Curated by PDSP Ki Database | J Pharmacol Exp Ther 289: 1151-9 (1999) BindingDB Entry DOI: 10.7270/Q2BZ64KX | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50070220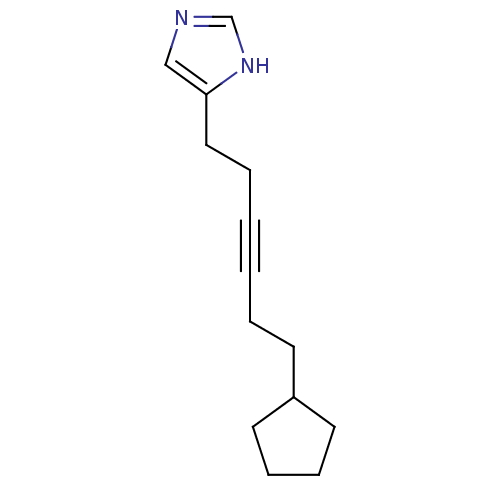 (4-(6-Cyclopentyl-hex-3-ynyl)-1H-imidazole | CHEMBL...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gliatech Inc. Curated by ChEMBL | Assay Description Binding affinity at histamine H3 receptor in rat cortical membranes by [3H]-Nalpha-methylhistamine displacement. | J Med Chem 42: 903-9 (1999) Article DOI: 10.1021/jm980310g BindingDB Entry DOI: 10.7270/Q2ST7P1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hrh3 protein (RAT) | BDBM50070220 (4-(6-Cyclopentyl-hex-3-ynyl)-1H-imidazole | CHEMBL...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gliatech Inc. Curated by PDSP Ki Database | J Pharmacol Exp Ther 289: 1151-9 (1999) BindingDB Entry DOI: 10.7270/Q2BZ64KX | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50070220 (4-(6-Cyclopentyl-hex-3-ynyl)-1H-imidazole | CHEMBL...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gliatech Inc. Curated by ChEMBL | Assay Description Inhibition of [3H]- N-alpha-methylhistamine from histamine H3 receptor | Bioorg Med Chem Lett 8: 1133-8 (1999) BindingDB Entry DOI: 10.7270/Q2GF0SPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM17231 (3-chloro-N-{4-chloro-2-[(4-chlorophenyl)carbamoyl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.950 | -51.0 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Berlex | Assay Description The enzyme activities were determined kinetically as the initial rate of cleavage of a peptide p-nitroanilide. Km for enzyme and substrate was determ... | Bioorg Med Chem 15: 2127-46 (2007) Article DOI: 10.1016/j.bmc.2006.12.019 BindingDB Entry DOI: 10.7270/Q2MS3R1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Cavia porcellus) | BDBM50038792 (6-Benzyloxy-3-((R)-2-pyridin-3-yl-thiazolidine-4-c...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Antagonist activity against platelet activating factor (PAF) receptor in rabbit platelet membranes using [3H]C18-PAF as radioligand | J Med Chem 37: 2011-32 (1994) BindingDB Entry DOI: 10.7270/Q2GH9JM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Cavia porcellus) | BDBM50038750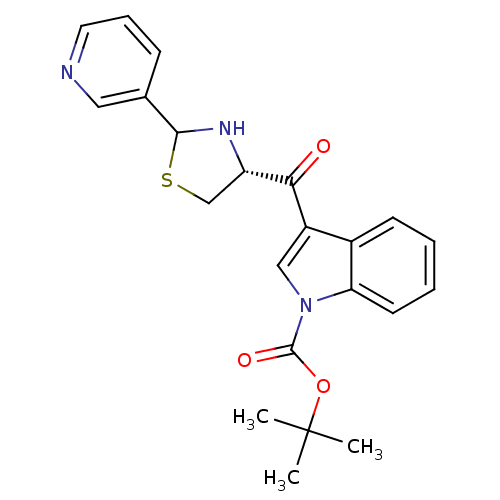 (3-((R)-2-Pyridin-3-yl-thiazolidine-4-carbonyl)-ind...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Antagonist activity against platelet activating factor (PAF) receptor in rabbit platelet membranes using [3H]C18-PAF as radioligand | J Med Chem 37: 2011-32 (1994) BindingDB Entry DOI: 10.7270/Q2GH9JM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Cavia porcellus) | BDBM50038806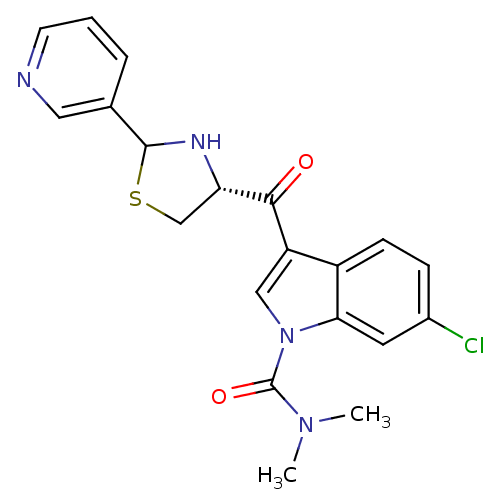 (6-Chloro-3-((R)-2-pyridin-3-yl-thiazolidine-4-carb...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Antagonist activity against platelet activating factor (PAF) receptor in rabbit platelet membranes using [3H]C18-PAF as radioligand | J Med Chem 37: 2011-32 (1994) BindingDB Entry DOI: 10.7270/Q2GH9JM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Cavia porcellus) | BDBM50038834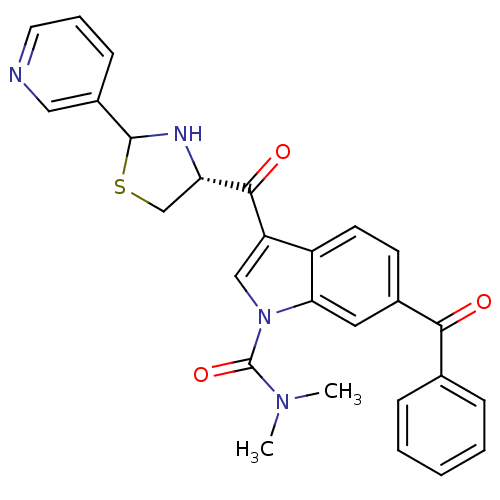 (6-Benzoyl-3-((R)-2-pyridin-3-yl-thiazolidine-4-car...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Antagonist activity against platelet activating factor (PAF) receptor in rabbit platelet membranes using [3H]C18-PAF as radioligand | J Med Chem 37: 2011-32 (1994) BindingDB Entry DOI: 10.7270/Q2GH9JM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 6011 total ) | Next | Last >> |