| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Chymotrypsinogen A |
|---|
| Ligand | BDBM222139 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | α-Chymotrypsin Inhibition Kinetic Assay |
|---|
| Ki | 2.6e+3± 2e+2 nM |
|---|
| Citation |  Marasini, BP; Rahim, F; Perveen, S; Karim, A; Mohammed Khan, K; Atta-Ur-Rahman, null; Choudhary, MI Synthesis, structure-activity relationships studies of benzoxazinone derivatives as a-chymotrypsin inhibitors. Bioorg Chem70:210-221 (2017) [PubMed] Article Marasini, BP; Rahim, F; Perveen, S; Karim, A; Mohammed Khan, K; Atta-Ur-Rahman, null; Choudhary, MI Synthesis, structure-activity relationships studies of benzoxazinone derivatives as a-chymotrypsin inhibitors. Bioorg Chem70:210-221 (2017) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Chymotrypsinogen A |
|---|
| Name: | Chymotrypsinogen A |
|---|
| Synonyms: | Alpha-chymotrypsin | CTRA_BOVIN | Chymotrypsin A | Chymotrypsin A chain A | Chymotrypsin A chain B | Chymotrypsin A chain C | Chymotrypsinogen A | alpha-Chymotrypsin (α-Chymotrypsin) |
|---|
| Type: | Serine protease |
|---|
| Mol. Mass.: | 25670.88 |
|---|
| Organism: | Bos taurus (bovine) |
|---|
| Description: | n/a |
|---|
| Residue: | 245 |
|---|
| Sequence: | CGVPAIQPVLSGLSRIVNGEEAVPGSWPWQVSLQDKTGFHFCGGSLINENWVVTAAHCGV
TTSDVVVAGEFDQGSSSEKIQKLKIAKVFKNSKYNSLTINNDITLLKLSTAASFSQTVSA
VCLPSASDDFAAGTTCVTTGWGLTRYTNANTPDRLQQASLPLLSNTNCKKYWGTKIKDAM
ICAGASGVSSCMGDSGGPLVCKKNGAWTLVGIVSWGSSTCSTSTPGVYARVTALVNWVQQ
TLAAN
|
|
|
|---|
| BDBM222139 |
|---|
| n/a |
|---|
| Name | BDBM222139 |
|---|
| Synonyms: | Chymostatin | US11859014, Compound chymostatin |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C31H41N7O6 |
|---|
| Mol. Mass. | 607.7005 |
|---|
| SMILES | CC(C)C[C@H](NC(=O)[C@@H](NC(=O)N[C@@H](Cc1ccccc1)C(O)=O)C1CCN=C(N)N1)C(=O)N[C@@H](Cc1ccccc1)C=O |t:28| |
|---|
| Structure | 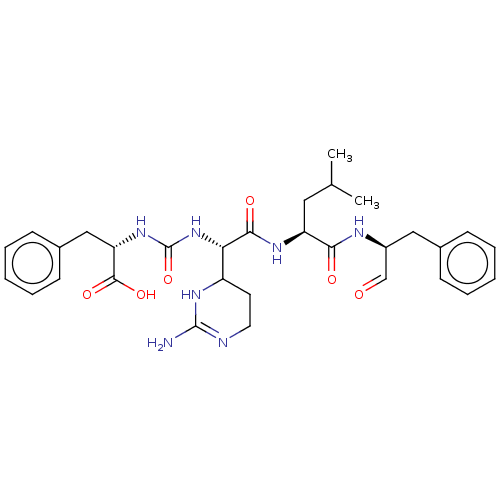
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Marasini, BP; Rahim, F; Perveen, S; Karim, A; Mohammed Khan, K; Atta-Ur-Rahman, null; Choudhary, MI Synthesis, structure-activity relationships studies of benzoxazinone derivatives as a-chymotrypsin inhibitors. Bioorg Chem70:210-221 (2017) [PubMed] Article
Marasini, BP; Rahim, F; Perveen, S; Karim, A; Mohammed Khan, K; Atta-Ur-Rahman, null; Choudhary, MI Synthesis, structure-activity relationships studies of benzoxazinone derivatives as a-chymotrypsin inhibitors. Bioorg Chem70:210-221 (2017) [PubMed] Article