| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Receptor-type tyrosine-protein phosphatase mu |
|---|
| Ligand | BDBM50054344 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | Inhibition Assay |
|---|
| IC50 | 3300±200 nM |
|---|
| Citation |  Zhang, Z; Zeng, L Hydroxyindole carboxylic acid based inhibitors for oncogenic Src homology-2 domain containing protein tyrosine phosphatase-2 (SHP2) US Patent US9522881 Publication Date 12/20/2016 Zhang, Z; Zeng, L Hydroxyindole carboxylic acid based inhibitors for oncogenic Src homology-2 domain containing protein tyrosine phosphatase-2 (SHP2) US Patent US9522881 Publication Date 12/20/2016 |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Receptor-type tyrosine-protein phosphatase mu |
|---|
| Name: | Receptor-type tyrosine-protein phosphatase mu |
|---|
| Synonyms: | PTPRL1 | PTPRM | PTPRM_HUMAN | Protein-tyrosine phosphatase mu (PTPmu) | Receptor-type tyrosine-protein phosphatase mu | Receptor-type tyrosine-protein phosphatase mu (PTPμ) |
|---|
| Type: | Protein |
|---|
| Mol. Mass.: | 163683.56 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P28827 |
|---|
| Residue: | 1452 |
|---|
| Sequence: | MRGLGTCLATLAGLLLTAAGETFSGGCLFDEPYSTCGYSQSEGDDFNWEQVNTLTKPTSD
PWMPSGSFMLVNASGRPEGQRAHLLLPQLKENDTHCIDFHYFVSSKSNSPPGLLNVYVKV
NNGPLGNPIWNISGDPTRTWNRAELAISTFWPNFYQVIFEVITSGHQGYLAIDEVKVLGH
PCTRTPHFLRIQNVEVNAGQFATFQCSAIGRTVAGDRLWLQGIDVRDAPLKEIKVTSSRR
FIASFNVVNTTKRDAGKYRCMIRTEGGVGISNYAELVVKEPPVPIAPPQLASVGATYLWI
QLNANSINGDGPIVAREVEYCTASGSWNDRQPVDSTSYKIGHLDPDTEYEISVLLTRPGE
GGTGSPGPALRTRTKCADPMRGPRKLEVVEVKSRQITIRWEPFGYNVTRCHSYNLTVHYC
YQVGGQEQVREEVSWDTENSHPQHTITNLSPYTNVSVKLILMNPEGRKESQELIVQTDED
LPGAVPTESIQGSTFEEKIFLQWREPTQTYGVITLYEITYKAVSSFDPEIDLSNQSGRVS
KLGNETHFLFFGLYPGTTYSFTIRASTAKGFGPPATNQFTTKISAPSMPAYELETPLNQT
DNTVTVMLKPAHSRGAPVSVYQIVVEEERPRRTKKTTEILKCYPVPIHFQNASLLNSQYY
FAAEFPADSLQAAQPFTIGDNKTYNGYWNTPLLPYKSYRIYFQAASRANGETKIDCVQVA
TKGAATPKPVPEPEKQTDHTVKIAGVIAGILLFVIIFLGVVLVMKKRKLAKKRKETMSST
RQEMTVMVNSMDKSYAEQGTNCDEAFSFMDTHNLNGRSVSSPSSFTMKTNTLSTSVPNSY
YPDETHTMASDTSSLVQSHTYKKREPADVPYQTGQLHPAIRVADLLQHITQMKCAEGYGF
KEEYESFFEGQSAPWDSAKKDENRMKNRYGNIIAYDHSRVRLQTIEGDTNSDYINGNYID
GYHRPNHYIATQGPMQETIYDFWRMVWHENTASIIMVTNLVEVGRVKCCKYWPDDTEIYK
DIKVTLIETELLAEYVIRTFAVEKRGVHEIREIRQFHFTGWPDHGVPYHATGLLGFVRQV
KSKSPPSAGPLVVHCSAGAGRTGCFIVIDIMLDMAEREGVVDIYNCVRELRSRRVNMVQT
EEQYVFIHDAILEACLCGDTSVPASQVRSLYYDMNKLDPQTNSSQIKEEFRTLNMVTPTL
RVEDCSIALLPRNHEKNRCMDILPPDRCLPFLITIDGESSNYINAALMDSYKQPSAFIVT
QHPLPNTVKDFWRLVLDYHCTSVVMLNDVDPAQLCPQYWPENGVHRHGPIQVEFVSADLE
EDIISRIFRIYNAARPQDGYRMVQQFQFLGWPMYRDTPVSKRSFLKLIRQVDKWQEEYNG
GEGRTVVHCLNGGGRSGTFCAISIVCEMLRHQRTVDVFHAVKTLRNNKPNMVDLLDQYKF
CYEVALEYLNSG
|
|
|
|---|
| BDBM50054344 |
|---|
| n/a |
|---|
| Name | BDBM50054344 |
|---|
| Synonyms: | CHEMBL3319356 | US9522881, 11a-1 L97M74 | US9844535, ID 11a-1 L97M74 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C28H20IN3O5S |
|---|
| Mol. Mass. | 637.445 |
|---|
| SMILES | Cn1c(c(I)c2cc(C(O)=O)c(O)cc12)-c1cccc(NC(=O)C(=O)Nc2ccc(cc2)-c2ccsc2)c1 |
|---|
| Structure | 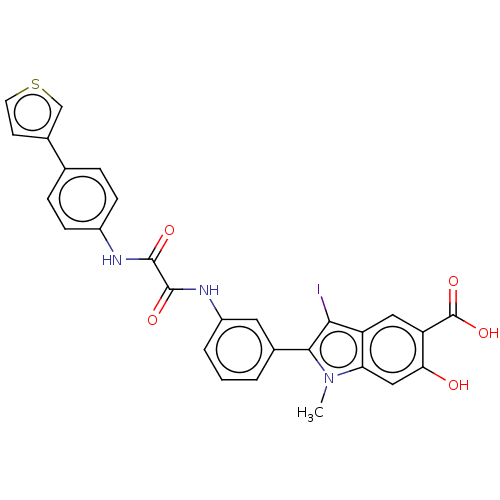
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Zhang, Z; Zeng, L Hydroxyindole carboxylic acid based inhibitors for oncogenic Src homology-2 domain containing protein tyrosine phosphatase-2 (SHP2) US Patent US9522881 Publication Date 12/20/2016
Zhang, Z; Zeng, L Hydroxyindole carboxylic acid based inhibitors for oncogenic Src homology-2 domain containing protein tyrosine phosphatase-2 (SHP2) US Patent US9522881 Publication Date 12/20/2016