| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Acetyl-CoA carboxylase 1 |
|---|
| Ligand | BDBM459208 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | Kinase Assay |
|---|
| IC50 | 0.880±n/a nM |
|---|
| Citation |  Tang, W; Yang, X; Gu, Z; Li, C; Zhang, Z; Wan, Z; Wang, X; Zhang, Y Thienopyrimidine derivative and use thereof in medicine US Patent US10759812 Publication Date 9/1/2020 Tang, W; Yang, X; Gu, Z; Li, C; Zhang, Z; Wan, Z; Wang, X; Zhang, Y Thienopyrimidine derivative and use thereof in medicine US Patent US10759812 Publication Date 9/1/2020 |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Acetyl-CoA carboxylase 1 |
|---|
| Name: | Acetyl-CoA carboxylase 1 |
|---|
| Synonyms: | ACAC | ACACA | ACACA_HUMAN | ACC-alpha | ACC1 | ACCA | Acetyl-CoA carboxylase 1 | Acetyl-CoA carboxylase 1 (ACC1) |
|---|
| Type: | Protein |
|---|
| Mol. Mass.: | 265543.22 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | Q13085 |
|---|
| Residue: | 2346 |
|---|
| Sequence: | MDEPSPLAQPLELNQHSRFIIGSVSEDNSEDEISNLVKLDLLEEKEGSLSPASVGSDTLS
DLGISSLQDGLALHIRSSMSGLHLVKQGRDRKKIDSQRDFTVASPAEFVTRFGGNKVIEK
VLIANNGIAAVKCMRSIRRWSYEMFRNERAIRFVVMVTPEDLKANAEYIKMADHYVPVPG
GPNNNNYANVELILDIAKRIPVQAVWAGWGHASENPKLPELLLKNGIAFMGPPSQAMWAL
GDKIASSIVAQTAGIPTLPWSGSGLRVDWQENDFSKRILNVPQELYEKGYVKDVDDGLQA
AEEVGYPVMIKASEGGGGKGIRKVNNADDFPNLFRQVQAEVPGSPIFVMRLAKQSRHLEV
QILADQYGNAISLFGRDCSVQRRHQKIIEEAPATIATPAVFEHMEQCAVKLAKMVGYVSA
GTVEYLYSQDGSFYFLELNPRLQVEHPCTEMVADVNLPAAQLQIAMGIPLYRIKDIRMMY
GVSPWGDSPIDFEDSAHVPCPRGHVIAARITSENPDEGFKPSSGTVQELNFRSNKNVWGY
FSVAAAGGLHEFADSQFGHCFSWGENREEAISNMVVALKELSIRGDFRTTVEYLIKLLET
ESFQMNRIDTGWLDRLIAEKVQAERPDTMLGVVCGALHVADVSLRNSVSNFLHSLERGQV
LPAHTLLNTVDVELIYEGVKYVLKVTRQSPNSYVVIMNGSCVEVDVHRLSDGGLLLSYDG
SSYTTYMKEEVDRYRITIGNKTCVFEKENDPSVMRSPSAGKLIQYIVEDGGHVFAGQCYA
EIEVMKMVMTLTAVESGCIHYVKRPGAALDPGCVLAKMQLDNPSKVQQAELHTGSLPRIQ
STALRGEKLHRVFHYVLDNLVNVMNGYCLPDPFFSSKVKDWVERLMKTLRDPSLPLLELQ
DIMTSVSGRIPPNVEKSIKKEMAQYASNITSVLCQFPSQQIANILDSHAATLNRKSEREV
FFMNTQSIVQLVQRYRSGIRGHMKAVVMDLLRQYLRVETQFQNGHYDKCVFALREENKSD
MNTVLNYIFSHAQVTKKNLLVTMLIDQLCGRDPTLTDELLNILTELTQLSKTTNAKVALR
ARQVLIASHLPSYELRHNQVESIFLSAIDMYGHQFCIENLQKLILSETSIFDVLPNFFYH
SNQVVRMAALEVYVRRAYIAYELNSVQHRQLKDNTCVVEFQFMLPTSHPNRGNIPTLNRM
SFSSNLNHYGMTHVASVSDVLLDNSFTPPCQRMGGMVSFRTFEDFVRIFDEVMGCFSDSP
PQSPTFPEAGHTSLYDEDKVPRDEPIHILNVAIKTDCDIEDDRLAAMFREFTQQNKATLV
DHGIRRLTFLVAQKDFRKQVNYEVDRRFHREFPKFFTFRARDKFEEDRIYRHLEPALAFQ
LELNRMRNFDLTAIPCANHKMHLYLGAAKVEVGTEVTDYRFFVRAIIRHSDLVTKEASFE
YLQNEGERLLLEAMDELEVAFNNTNVRTDCNHIFLNFVPTVIMDPSKIEESVRSMVMRYG
SRLWKLRVLQAELKINIRLTPTGKAIPIRLFLTNESGYYLDISLYKEVTDSRTAQIMFQA
YGDKQGPLHGMLINTPYVTKDLLQSKRFQAQSLGTTYIYDIPEMFRQSLIKLWESMSTQA
FLPSPPLPSDMLTYTELVLDDQGQLVHMNRLPGGNEIGMVAWKMTFKSPEYPEGRDIIVI
GNDITYRIGSFGPQEDLLFLRASELARAEGIPRIYVSANSGARIGLAEEIRHMFHVAWVD
PEDPYKGYRYLYLTPQDYKRVSALNSVHCEHVEDEGESRYKITDIIGKEEGIGPENLRGS
GMIAGESSLAYNEIITISLVTCRAIGIGAYLVRLGQRTIQVENSHLILTGAGALNKVLGR
EVYTSNNQLGGIQIMHNNGVTHCTVCDDFEGVFTVLHWLSYMPKSVHSSVPLLNSKDPID
RIIEFVPTKTPYDPRWMLAGRPHPTQKGQWLSGFFDYGSFSEIMQPWAQTVVVGRARLGG
IPVGVVAVETRTVELSIPADPANLDSEAKIIQQAGQVWFPDSAFKTYQAIKDFNREGLPL
MVFANWRGFSGGMKDMYDQVLKFGAYIVDGLRECCQPVLVYIPPQAELRGGSWVVIDSSI
NPRHMEMYADRESRGSVLEPEGTVEIKFRRKDLVKTMRRVDPVYIHLAERLGTPELSTAE
RKELENKLKEREEFLIPIYHQVAVQFADLHDTPGRMQEKGVISDILDWKTSRTFFYWRLR
RLLLEDLVKKKIHNANPELTDGQIQAMLRRWFVEVEGTVKAYVWDNNKDLAEWLEKQLTE
EDGVHSVIEENIKCISRDYVLKQIRSLVQANPEVAMDSIIHMTQHISPTQRAEVIRILST
MDSPST
|
|
|
|---|
| BDBM459208 |
|---|
| n/a |
|---|
| Name | BDBM459208 |
|---|
| Synonyms: | 2-[1-[2-[[(3aR,6aS)-5-ethyl-5-hydroxy-2,3,3a,4,6,6a-hexahydro-1H-pentalen-2-yl]oxy]-2-(2-methoxyphenyl)ethyl)-5-methyl-6-oxazol-2-yl-2,4-dioxo-thieno[2,3-d]pyrimid-3-yl]-2-methyl-propanoic acid | US10759812, Example 19 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C33H39N3O8S |
|---|
| Mol. Mass. | 637.743 |
|---|
| SMILES | CCC1(O)C[C@@H]2C[C@@H](C[C@@H]2C1)OC(Cn1c2sc(c(C)c2c(=O)n(c1=O)C(C)(C)C(O)=O)-c1ncco1)c1ccccc1OC |r| |
|---|
| Structure | 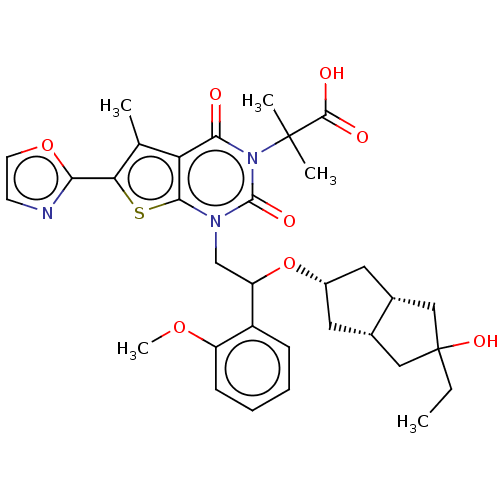
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Tang, W; Yang, X; Gu, Z; Li, C; Zhang, Z; Wan, Z; Wang, X; Zhang, Y Thienopyrimidine derivative and use thereof in medicine US Patent US10759812 Publication Date 9/1/2020
Tang, W; Yang, X; Gu, Z; Li, C; Zhang, Z; Wan, Z; Wang, X; Zhang, Y Thienopyrimidine derivative and use thereof in medicine US Patent US10759812 Publication Date 9/1/2020