null
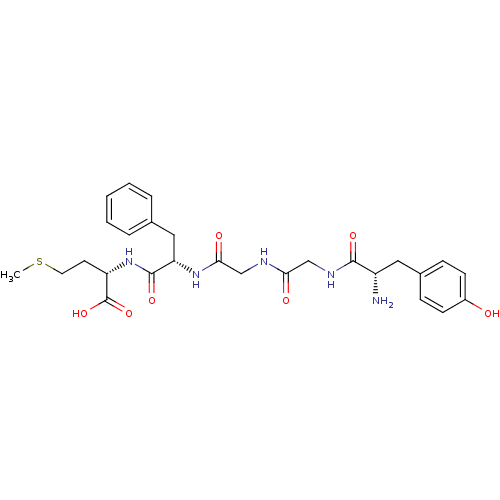
SMILES CSCC[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccc(O)cc1)C(O)=O
InChI Key InChIKey=YFGBQHOOROIVKG-FKBYEOEOSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50019056
Found 3 hits for monomerid = 50019056
TargetMu-type opioid receptor(Homo sapiens (Human))
University of Pennsylvania
Curated by PDSP Ki Database
University of Pennsylvania
Curated by PDSP Ki Database
TargetDelta-type opioid receptor(Homo sapiens (Human))
University of Pennsylvania
Curated by PDSP Ki Database
University of Pennsylvania
Curated by PDSP Ki Database
TargetKappa-type opioid receptor(Homo sapiens (Human))
University of Pennsylvania
Curated by PDSP Ki Database
University of Pennsylvania
Curated by PDSP Ki Database