null
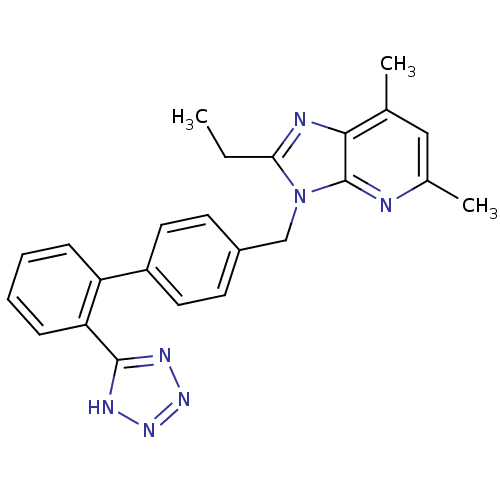
SMILES CCc1nc2c(C)cc(C)nc2n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1
InChI Key InChIKey=YFWXFHNZGKNDBC-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50009718
Found 2 hits for monomerid = 50009718
Affinity DataIC50: 2.70nMAssay Description:Displacement of [125I]Tyr4-Sar1,Ile8-Angiotensin II from human Angiotensin 1 receptor after 60 mins by scintillation countingMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor gamma(Homo sapiens (Human))
Pfizer Inc.
Curated by ChEMBL
Pfizer Inc.
Curated by ChEMBL
Affinity DataEC50: >2.00E+4nMAssay Description:Partial agonist activity at human PPARgamma-LBD/Gal4 DNA binding domain by transactivation assayMore data for this Ligand-Target Pair