null
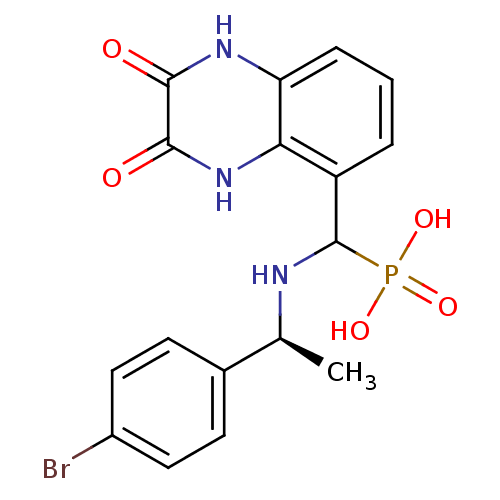
SMILES C[C@H](NC(c1cccc2[nH]c(=O)c(=O)[nH]c12)P(O)(O)=O)c1ccc(Br)cc1
InChI Key InChIKey=XXZGNAZRWCBSBK-WFVOFKTRSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 1 hit for monomerid = 50111396
Found 1 hit for monomerid = 50111396
Affinity DataKi: 11.6nMAssay Description:Inhibitory constant against (S)-glutamate and glycine evoked currents mediated by N-methyl-D-aspartate glutamate receptor NR1a/NR2C expressed in Xeno...More data for this Ligand-Target Pair