null
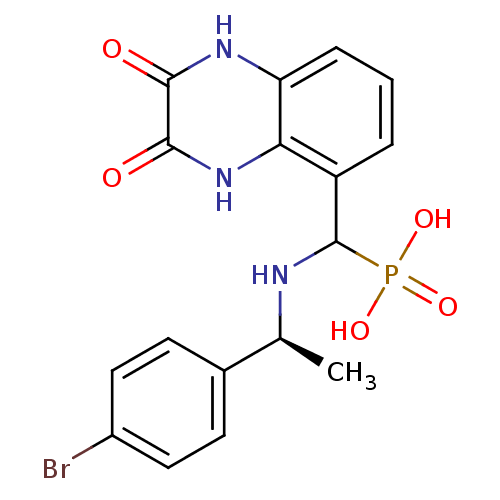
SMILES C[C@H](NC(c1cccc2[nH]c(=O)c(=O)[nH]c12)P(O)(O)=O)c1ccc(Br)cc1
InChI Key InChIKey=XXZGNAZRWCBSBK-WFVOFKTRSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 5 hits for monomerid = 50111396
Found 5 hits for monomerid = 50111396
TargetGlutamate receptor ionotropic, NMDA 1/2A(Rattus norvegicus (Rat))
University Walk
Curated by ChEMBL
University Walk
Curated by ChEMBL
Affinity DataKi: 5.40nMAssay Description:Inhibitory constant against (S)-glutamate and glycine evoked currents mediated by N-methyl-D-aspartate glutamate receptor NR1a/NR2A expressed in Xeno...More data for this Ligand-Target Pair
Affinity DataKi: 11.6nMAssay Description:Inhibitory constant against (S)-glutamate and glycine evoked currents mediated by N-methyl-D-aspartate glutamate receptor NR1a/NR2C expressed in Xeno...More data for this Ligand-Target Pair
TargetGlutamate receptor ionotropic, NMDA 1/2B(Rattus norvegicus (Rat))
University Walk
Curated by ChEMBL
University Walk
Curated by ChEMBL
Affinity DataKi: 67nMAssay Description:Inhibitory constant against (S)-glutamate and glycine evoked currents mediated by N-methyl-D-aspartate glutamate receptor NR1a/NR2B expressed in Xeno...More data for this Ligand-Target Pair
TargetGlutamate receptor ionotropic, NMDA 1/2A(Homo sapiens (Human))
Novartis Pharma AG
Curated by ChEMBL
Novartis Pharma AG
Curated by ChEMBL
Affinity DataIC50: 270nMAssay Description:Inhibitory activity against Xenopus laevis oocyte expressing 1A/2A heteromeric human NMDA (hNMDA) receptorMore data for this Ligand-Target Pair
TargetGlutamate receptor ionotropic, NMDA 1/2B(Homo sapiens (Human))
Novartis Pharma AG
Curated by ChEMBL
Novartis Pharma AG
Curated by ChEMBL
Affinity DataIC50: 2.96E+4nMAssay Description:Inhibitory activity against Xenopus laevis oocyte expressing 1A/2B heteromeric human NMDA (hNMDA) receptorMore data for this Ligand-Target Pair