null
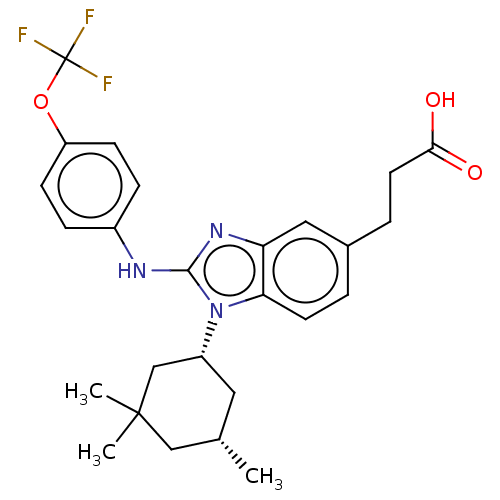
SMILES C[C@H]1C[C@H](CC(C)(C)C1)n1c(Nc2ccc(OC(F)(F)F)cc2)nc2cc(CCC(O)=O)ccc12
InChI Key InChIKey=RNMAUIMMNAHKQR-QFBILLFUSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 11 hits for monomerid = 404661
Found 11 hits for monomerid = 404661
TargetIsocitrate dehydrogenase [NADP] cytoplasmic(Homo sapiens (Human))
China Pharmaceutical University
Curated by ChEMBL
China Pharmaceutical University
Curated by ChEMBL
Affinity DataIC50: 15nMAssay Description:Inhibition of IDH1 R132C mutant (unknown origin) using alphaKG as substrate after 90 mins by fluorescence assayMore data for this Ligand-Target Pair
TargetIsocitrate dehydrogenase [NADP] cytoplasmic(Homo sapiens (Human))
China Pharmaceutical University
Curated by ChEMBL
China Pharmaceutical University
Curated by ChEMBL
Affinity DataIC50: 15nMAssay Description:Inhibition of IDH1 R132H mutant (unknown origin) using alphaKG as substrate after 90 mins by fluorescence assayMore data for this Ligand-Target Pair
TargetIsocitrate dehydrogenase [NADP] cytoplasmic [R132H](Homo sapiens (Human))
Bayer Pharma Aktiengesellschaft
US Patent
Bayer Pharma Aktiengesellschaft
US Patent
Affinity DataIC50: 20nMAssay Description:IDH1 R132H catalyzes the NADPH-dependent reduction of alpha-ketoglutarate (α-KG) to (2R)-2-hydroxyglutarate (2-HG). NADPH consumption is determi...More data for this Ligand-Target Pair
TargetIsocitrate dehydrogenase [NADP] cytoplasmic [R132H](Homo sapiens (Human))
Bayer Pharma Aktiengesellschaft
US Patent
Bayer Pharma Aktiengesellschaft
US Patent
Affinity DataIC50: 140nMAssay Description:The biochemical reactions were performed at 32° C. in 384-well plates using a reaction volume of 41 μL and the following assay buffer conditions...More data for this Ligand-Target Pair
TargetIsocitrate dehydrogenase [NADP] cytoplasmic(Homo sapiens (Human))
China Pharmaceutical University
Curated by ChEMBL
China Pharmaceutical University
Curated by ChEMBL
Affinity DataIC50: 10nMAssay Description:Inhibition of IDH1 R132H mutant (unknown origin) assessed as reduction in NADPH consumption using alpha-KG as substrate by by luminescence based assa...More data for this Ligand-Target Pair
Affinity DataIC50: 15nMAssay Description:Compound 1 was not initially reported as having the greatest biochemical potency compared to reports for certain other small molecule inhibitors of m...More data for this Ligand-Target Pair
Affinity DataIC50: 3.05E+3nMAssay Description:Please see paper.More data for this Ligand-Target Pair
TargetIsocitrate dehydrogenase [NADP] cytoplasmic [R132H](Homo sapiens (Human))
Bayer Pharma Aktiengesellschaft
US Patent
Bayer Pharma Aktiengesellschaft
US Patent
Affinity DataIC50: 15nMAssay Description:The cellular potency of Compound 1 in suppressing intracellular 2-HG levels was determined in cell lines expressing five different mutated IDH-1 prot...More data for this Ligand-Target Pair
Affinity DataIC50: 3.05E+3nMAssay Description:The cellular potency of Compound 1 in suppressing intracellular 2-HG levels was determined in cell lines expressing five different mutated IDH-1 prot...More data for this Ligand-Target Pair
TargetIsocitrate dehydrogenase [NADP] cytoplasmic [R132H](Homo sapiens (Human))
Bayer Pharma Aktiengesellschaft
US Patent
Bayer Pharma Aktiengesellschaft
US Patent
Affinity DataIC50: 15nMAssay Description:Compound 1 was not initially reported as having the greatest biochemical potency compared to reports for certain other small molecule inhibitors of m...More data for this Ligand-Target Pair
TargetIsocitrate dehydrogenase [NADP] cytoplasmic [R132H](Homo sapiens (Human))
Bayer Pharma Aktiengesellschaft
US Patent
Bayer Pharma Aktiengesellschaft
US Patent