null
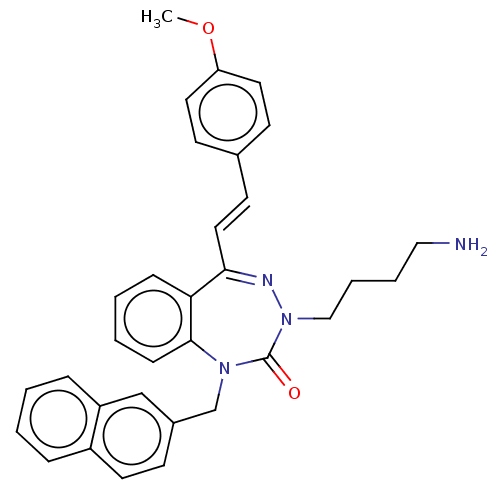
SMILES Cl.COc1ccc(\C=C\C2=NN(CCCCN)C(=O)N(Cc3ccc4ccccc4c3)c3ccccc23)cc1
InChI Key InChIKey=NKZTVWHDNWNDGG-XDJHFCHBSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50269945
Found 2 hits for monomerid = 50269945
Affinity DataEC50: 8.5nMAssay Description:Allosteric modulation of urotensin-2 receptor in Sprague-Dawley rat thoracic aorta assessed as change in URP-mediated aortic ring contraction by meas...More data for this Ligand-Target Pair
Affinity DataEC50: 3.60nMAssay Description:Allosteric modulation of urotensin-2 receptor in Sprague-Dawley rat thoracic aorta assessed as change in human urotensin-2-mediated aortic ring contr...More data for this Ligand-Target Pair