null
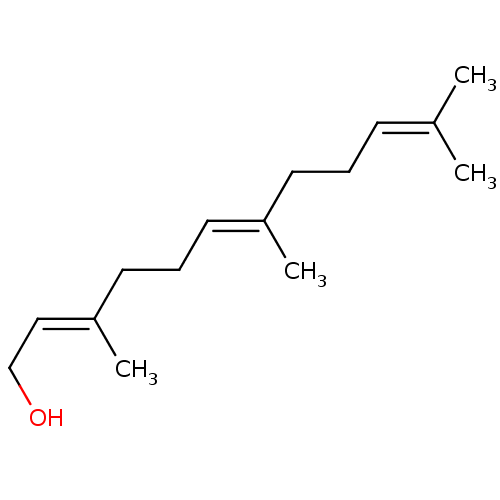
SMILES [#6]\[#6](-[#6])=[#6]\[#6]-[#6]\[#6](-[#6])=[#6]\[#6]-[#6]\[#6](-[#6])=[#6]\[#6]-[#8]
InChI Key InChIKey=CRDAMVZIKSXKFV-YFVJMOTDSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 10 hits for monomerid = 11021
Found 10 hits for monomerid = 11021
Affinity DataKi: 800nM ΔG°: -8.31kcal/molepH: 7.4 T: 2°CAssay Description:MAO A and MAO B activities were determined spectrophotometrically. Competitive Ki values for both enzymes were determined by measuring initial rates ...More data for this Ligand-Target Pair
Affinity DataKi: 2.30E+3nM ΔG°: -7.69kcal/molepH: 7.5 T: 2°CAssay Description:MAO A and MAO B activities were determined spectrophotometrically. Competitive Ki values for both enzymes were determined by measuring initial rates ...More data for this Ligand-Target Pair
Affinity DataKi: 2.40E+3nM ΔG°: -7.66kcal/molepH: 7.4 T: 2°CAssay Description:MAO A and MAO B activities were determined spectrophotometrically. Competitive Ki values for both enzymes were determined by measuring initial rates ...More data for this Ligand-Target Pair
Affinity DataKi: 5.00E+3nM ΔG°: -7.23kcal/molepH: 7.4 T: 2°CAssay Description:MAO A and MAO B activities were determined spectrophotometrically. Competitive Ki values for both enzymes were determined by measuring initial rates ...More data for this Ligand-Target Pair
Affinity DataKi: >1.00E+6nMAssay Description:MAO A and MAO B activities were determined spectrophotometrically. Competitive Ki values for both enzymes were determined by measuring initial rates ...More data for this Ligand-Target Pair
Affinity DataKi: >1.00E+6nM ΔG°: >-4.09kcal/molepH: 7.4 T: 2°CAssay Description:MAO A and MAO B activities were determined spectrophotometrically. Competitive Ki values for both enzymes were determined by measuring initial rates ...More data for this Ligand-Target Pair
Affinity DataKi: >1.00E+6nM ΔG°: >-4.09kcal/molepH: 7.5 T: 2°CAssay Description:MAO A and MAO B activities were determined spectrophotometrically. Competitive Ki values for both enzymes were determined by measuring initial rates ...More data for this Ligand-Target Pair
Affinity DataIC50: 4.00E+4nMAssay Description:Inhibition of human recombinant UGT1A1 expressed in HEK293 cells assessed as reduction in estradiol 3-glucuronidation by LC-MS/MS methodMore data for this Ligand-Target Pair
Affinity DataIC50: 4.70E+4nMAssay Description:Inhibition of human recombinant UGT1A1 expressed in HEK293 cells assessed as reduction in bilirubin glucuronidation by LC-MS/MS methodMore data for this Ligand-Target Pair
TargetNeuraminidase(Influenza A virus (A/Puerto Rico/8/34/Mount Sinai(...)
University of Innsbruck
Curated by ChEMBL
University of Innsbruck
Curated by ChEMBL
Affinity DataIC50: 8.14E+4nMAssay Description:Inhibition of Influenza Virus A/PR/8/34 neuraminidase chemiluminescence-based enzyme inhibition assayMore data for this Ligand-Target Pair
 3D Structure (crystal)
3D Structure (crystal)