null
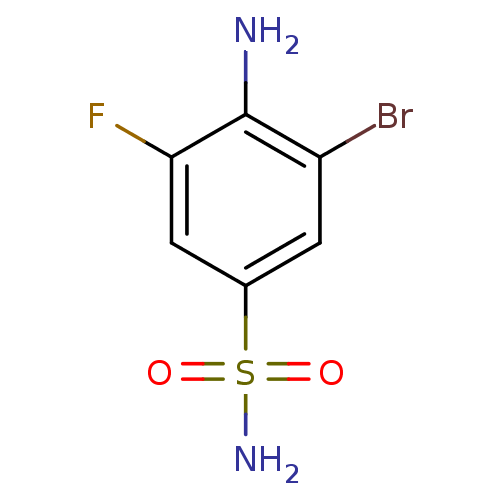
SMILES Nc1c(F)cc(cc1Br)S(N)(=O)=O
InChI Key InChIKey=GKYQTYGVQVABPW-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 7 hits for monomerid = 11606
Found 7 hits for monomerid = 11606
TargetCarbonic anhydrase 2(Homo sapiens (Human))
University of Agricultural Sciences and Veterinary Medicine
University of Agricultural Sciences and Veterinary Medicine
Affinity DataKi: 33nMAssay Description:An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic...More data for this Ligand-Target Pair
TargetCarbonic anhydrase 9(Homo sapiens (Human))
University of Agricultural Sciences and Veterinary Medicine
University of Agricultural Sciences and Veterinary Medicine
Affinity DataKi: 69nMAssay Description:An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic...More data for this Ligand-Target Pair
TargetCarbonic anhydrase 4(Bos taurus (bovine))
University of Agricultural Sciences and Veterinary Medicine
University of Agricultural Sciences and Veterinary Medicine
Affinity DataKi: 113nMAssay Description:An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic...More data for this Ligand-Target Pair
Affinity DataKi: 940nM ΔG°: -8.22kcal/molepH: 8.3 T: 2°CAssay Description:An Applied Photophysics stopped-flow instrument has been used for assaying the CA catalysed CO2 hydration activity [Khalifah et al., J. Biol. Chem., ...More data for this Ligand-Target Pair
Affinity DataKi: 3.19E+3nM ΔG°: -7.49kcal/molepH: 8.3 T: 2°CAssay Description:An Applied Photophysics stopped-flow instrument has been used for assaying the CA catalysed CO2 hydration activity [Khalifah et al., J. Biol. Chem., ...More data for this Ligand-Target Pair
TargetCarbonic anhydrase 1(Homo sapiens (Human))
University of Agricultural Sciences and Veterinary Medicine
University of Agricultural Sciences and Veterinary Medicine
Affinity DataKi: 3.90E+3nMAssay Description:An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic...More data for this Ligand-Target Pair
Affinity DataKi: 4.31E+3nM ΔG°: -7.31kcal/molepH: 8.3 T: 2°CAssay Description:An Applied Photophysics stopped-flow instrument has been used for assaying the CA catalysed CO2 hydration activity [Khalifah et al., J. Biol. Chem., ...More data for this Ligand-Target Pair