null
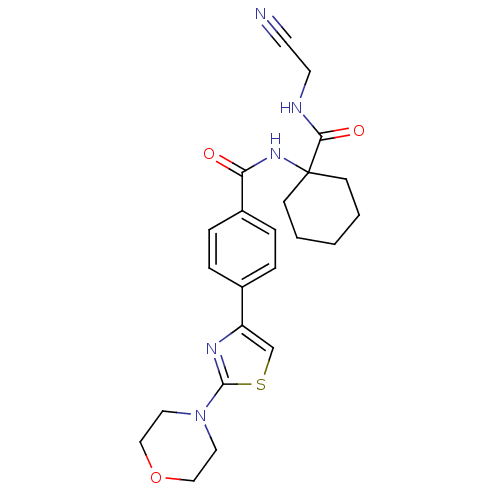
SMILES O=C(NC1(CCCCC1)C(=O)NCC#N)c1ccc(cc1)-c1csc(n1)N1CCOCC1
InChI Key InChIKey=TYJLGELUBBAGKK-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 7 hits for monomerid = 19857
Found 7 hits for monomerid = 19857
Affinity DataKi: 2.30nMAssay Description:Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 2.40E+4nM EC50: >1.00E+4nMpH: 5.5 T: 2°CAssay Description:Prior to the addition of substrate, different concentrations of the inhibitor ranging from 100 uM to 0.2 nM were preincubated for 15 min with enzyme ...More data for this Ligand-Target Pair
Affinity DataIC50: 4.20E+3nM EC50: 2.90E+3nMpH: 6.0 T: 2°CAssay Description:Prior to the addition of substrate, different concentrations of the inhibitor ranging from 100 uM to 0.2 nM were preincubated for 15 min with enzyme ...More data for this Ligand-Target Pair
Affinity DataIC50: 9.5nM EC50: 41nMpH: 5.5 T: 2°CAssay Description:Prior to the addition of substrate, different concentrations of the inhibitor ranging from 100 uM to 0.2 nM were preincubated for 15 min with enzyme ...More data for this Ligand-Target Pair
Affinity DataIC50: 8.50E+3nM EC50: 6.70E+3nMAssay Description:Prior to the addition of substrate, different concentrations of the inhibitor ranging from 100 uM to 0.2 nM were preincubated for 15 min with enzyme ...More data for this Ligand-Target Pair
Affinity DataIC50: 2.30nMpH: 5.5 T: 2°CAssay Description:Prior to the addition of substrate, different concentrations of the inhibitor ranging from 100 uM to 0.2 nM were preincubated for 15 min with enzyme ...More data for this Ligand-Target Pair
Affinity DataIC50: 2.70E+4nM EC50: >1.00E+4nMpH: 6.5 T: 2°CAssay Description:Prior to the addition of substrate, different concentrations of the inhibitor ranging from 100 uM to 0.2 nM were preincubated for 15 min with enzyme ...More data for this Ligand-Target Pair