null
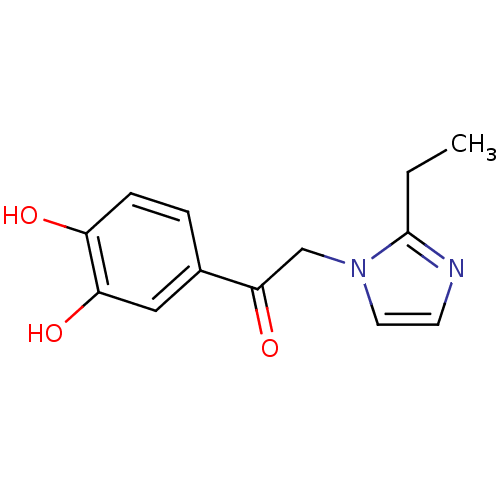
SMILES CCc1nccn1CC(=O)c1ccc(O)c(O)c1
InChI Key InChIKey=KRFQWVXRVPHWCH-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 8 hits for monomerid = 41888
Found 8 hits for monomerid = 41888
TargetAlkaline phosphatase, germ cell type(Homo sapiens (Human))
Sanford-Burnham Center for Chemical Genomics
Curated by PubChem BioAssay
Sanford-Burnham Center for Chemical Genomics
Curated by PubChem BioAssay
Affinity DataIC50: 1.00E+5nMAssay Description:Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C...More data for this Ligand-Target Pair
TargetAlkaline phosphatase, tissue-nonspecific isozyme(Homo sapiens (Human))
Burnham Center for Chemical Genomics
Curated by PubChem BioAssay
Burnham Center for Chemical Genomics
Curated by PubChem BioAssay
Affinity DataIC50: 6.25E+4nMAssay Description:Sanford-Burnham Center for Chemical Genomics (SBCCG) Sanford-Burnham Medical Research Institute (SBMRI, San Diego, CA) NIH Molecular Libraries Screen...More data for this Ligand-Target Pair
TargetAlkaline phosphatase, germ cell type(Homo sapiens (Human))
Sanford-Burnham Center for Chemical Genomics
Curated by PubChem BioAssay
Sanford-Burnham Center for Chemical Genomics
Curated by PubChem BioAssay
Affinity DataIC50: 3.73E+3nMAssay Description:Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C...More data for this Ligand-Target Pair
TargetPhospholipase A-2-activating protein(Homo sapiens (Human))
Human BioMolecular Research Institute
Curated by ChEMBL
Human BioMolecular Research Institute
Curated by ChEMBL
Affinity DataIC50: 4.20E+3nMAssay Description:Inhibition of PLAP by analogous luminescence assayMore data for this Ligand-Target Pair
TargetPhospholipase A-2-activating protein(Homo sapiens (Human))
Human BioMolecular Research Institute
Curated by ChEMBL
Human BioMolecular Research Institute
Curated by ChEMBL
Affinity DataIC50: 4.20E+3nMAssay Description:Inhibition of PLAP by analogous luminescence assayMore data for this Ligand-Target Pair
TargetAlkaline phosphatase, tissue-nonspecific isozyme(Homo sapiens (Human))
Burnham Center for Chemical Genomics
Curated by PubChem BioAssay
Burnham Center for Chemical Genomics
Curated by PubChem BioAssay
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of TNAP by analogous luminescence assayMore data for this Ligand-Target Pair
TargetIntestinal-type alkaline phosphatase(Homo sapiens (Human))
Human BioMolecular Research Institute
Curated by ChEMBL
Human BioMolecular Research Institute
Curated by ChEMBL
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of IAP by analogous luminescence assayMore data for this Ligand-Target Pair
TargetAlkaline phosphatase, germ cell type(Homo sapiens (Human))
Sanford-Burnham Center for Chemical Genomics
Curated by PubChem BioAssay
Sanford-Burnham Center for Chemical Genomics
Curated by PubChem BioAssay
Affinity DataIC50: 1.70E+3nMAssay Description:Inhibition of GCAP by analogous luminescence assayMore data for this Ligand-Target Pair