null
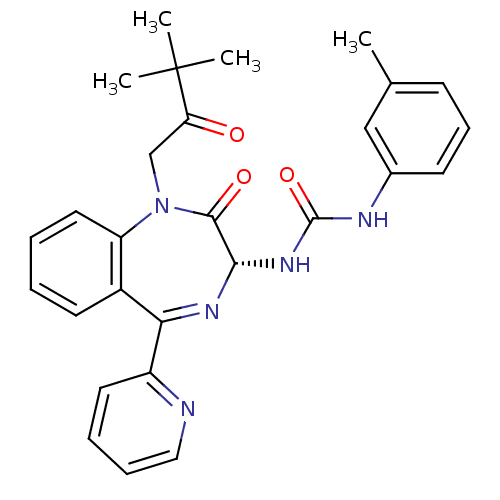
SMILES Cc1cccc(NC(=O)N[C@@H]2N=C(c3ccccn3)c3ccccc3N(CC(=O)C(C)(C)C)C2=O)c1
InChI Key InChIKey=OCWLXZANZZFWCX-VWLOTQADSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50056096
Found 4 hits for monomerid = 50056096
Affinity DataIC50: 470nMAssay Description:Inhibitory concentration against radioligand [3 H]L-364,718 binding to gastrin/Cholecystokinin type A receptor from rat pancreasMore data for this Ligand-Target Pair
Affinity DataIC50: 470nMAssay Description:Binding affinity towards Cholecystokinin type A receptor from rat pancreas using [I125]-L-364,718 as the radioligandMore data for this Ligand-Target Pair
Affinity DataIC50: 0.440nMAssay Description:Binding affinity towards gastrin/Cholecystokinin type B receptor from rat brain using [125I]-CCK-8 as the radioligandMore data for this Ligand-Target Pair
Affinity DataIC50: 0.440nMAssay Description:Inhibitory concentration against radioligand [125I]-CCK-8 binding to gastrin/Cholecystokinin type B receptor from rat brainMore data for this Ligand-Target Pair