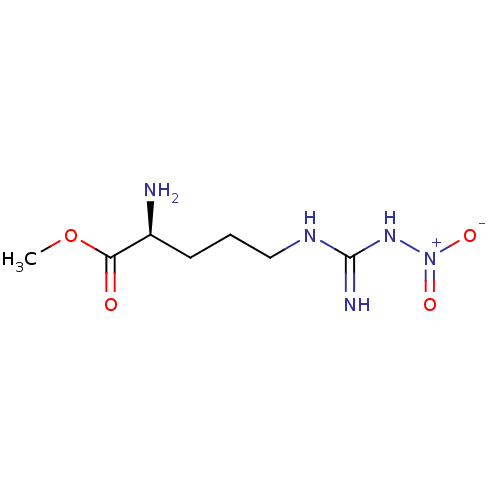
null
SMILES COC(=O)[C@@H](N)CCCNC(N)=N[N+]([O-])=O
InChI Key InChIKey=KCWZGJVSDFYRIX-YFKPBYRVSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 21 hits for monomerid = 50098937
Found 21 hits for monomerid = 50098937
TargetNitric oxide synthase, inducible(Homo sapiens (Human))
AstraZeneca R&D Charnwood
Curated by ChEMBL
AstraZeneca R&D Charnwood
Curated by ChEMBL
Affinity DataIC50: 1.40E+4nMAssay Description:Ability to inhibit conversion of [3H]-L-Arg to [3H]-L-citrulline catalyzed by inducible NOS (i NOS) from human DLD-1 cellsMore data for this Ligand-Target Pair
TargetNitric oxide synthase, inducible(Homo sapiens (Human))
AstraZeneca R&D Charnwood
Curated by ChEMBL
AstraZeneca R&D Charnwood
Curated by ChEMBL
Affinity DataIC50: 3.00E+8nMAssay Description:Inhibitory concentration against nitric oxide synthesis in intact DLD-1 cellsMore data for this Ligand-Target Pair
TargetNitric oxide synthase, endothelial(Homo sapiens (Human))
AstraZeneca R&D Charnwood
Curated by ChEMBL
AstraZeneca R&D Charnwood
Curated by ChEMBL
Affinity DataIC50: 2.70E+3nMAssay Description:Ability to inhibit conversion of [3H]-L-Arg to [3H]-L-citrulline catalyzed by endothelial NOS (e NOS) from HUVEC cellsMore data for this Ligand-Target Pair
TargetNitric oxide synthase, inducible(Homo sapiens (Human))
AstraZeneca R&D Charnwood
Curated by ChEMBL
AstraZeneca R&D Charnwood
Curated by ChEMBL
Affinity DataIC50: 8.00E+3nMAssay Description:In vitro inhibition of inducible nitric oxide synthase.More data for this Ligand-Target Pair
Affinity DataIC50: 1.60E+3nMAssay Description:Concentration required to inhibit neuronal nitric oxide synthaseMore data for this Ligand-Target Pair
TargetNitric oxide synthase, endothelial(Homo sapiens (Human))
AstraZeneca R&D Charnwood
Curated by ChEMBL
AstraZeneca R&D Charnwood
Curated by ChEMBL
Affinity DataIC50: 1.50E+3nMAssay Description:In vitro inhibition of endothelial nitric oxide synthase.More data for this Ligand-Target Pair
TargetNitric oxide synthase, inducible(Mus musculus (mouse))
National Cheng Kung University
Curated by ChEMBL
National Cheng Kung University
Curated by ChEMBL
Affinity DataIC50: 1.34E+4nMAssay Description:Inhibition of iNOS-mediated nitric oxide production in LPS-stimulated mouse BV2 cells measured after 24 hrs of post-stimulation by Griess reaction me...More data for this Ligand-Target Pair
TargetNitric oxide synthase, inducible(Homo sapiens (Human))
AstraZeneca R&D Charnwood
Curated by ChEMBL
AstraZeneca R&D Charnwood
Curated by ChEMBL
Affinity DataIC50: 8.00E+3nMAssay Description:Inhibitory concentration against human Inducible nitric oxide synthaseMore data for this Ligand-Target Pair
Affinity DataIC50: 1.60E+3nMAssay Description:Inhibitory concentration against recombinant human (neuronal nitric oxide synthase) n-NOSMore data for this Ligand-Target Pair
TargetNitric oxide synthase, endothelial(Homo sapiens (Human))
AstraZeneca R&D Charnwood
Curated by ChEMBL
AstraZeneca R&D Charnwood
Curated by ChEMBL
Affinity DataIC50: 1.50E+3nMAssay Description:Inhibitory concentration against recombinant human Endothelial nitric oxide synthaseMore data for this Ligand-Target Pair
TargetNitric oxide synthase, endothelial(Bos taurus (bovine))
University of Michigan
Curated by ChEMBL
University of Michigan
Curated by ChEMBL
Affinity DataIC50: 1.00E+5nMAssay Description:Compound was tested for for its inhibitory activity against endothelial NO2- synthesisMore data for this Ligand-Target Pair
TargetNitric oxide synthase, inducible(Mus musculus (mouse))
National Cheng Kung University
Curated by ChEMBL
National Cheng Kung University
Curated by ChEMBL
Affinity DataIC50: 2.60E+3nMAssay Description:Compound was tested for for its inhibitory activity against macrophage NO2- synthesisMore data for this Ligand-Target Pair
TargetNitric oxide synthase, endothelial(Homo sapiens (Human))
AstraZeneca R&D Charnwood
Curated by ChEMBL
AstraZeneca R&D Charnwood
Curated by ChEMBL
Affinity DataIC50: 2.70E+3nMAssay Description:Inhibitory activity against human endothelial nitiric oxide synthaseMore data for this Ligand-Target Pair
TargetNitric oxide synthase, inducible(Homo sapiens (Human))
AstraZeneca R&D Charnwood
Curated by ChEMBL
AstraZeneca R&D Charnwood
Curated by ChEMBL
Affinity DataIC50: 1.40E+4nMAssay Description:Inhibitory activity of compound against human inducible nitiric oxide synthaseMore data for this Ligand-Target Pair
Affinity DataIC50: 150nMAssay Description:Inhibitory activity against human neuronal nitiric oxide synthaseMore data for this Ligand-Target Pair
TargetNitric oxide synthase, inducible(Mus musculus (mouse))
National Cheng Kung University
Curated by ChEMBL
National Cheng Kung University
Curated by ChEMBL
Affinity DataIC50: 2.58E+4nMAssay Description:Inhibition of iNOS-mediated NO production in LPS-induced mouse BV2 cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 690nMAssay Description:Inhibition of human recombinant nNOS expressed in Sf9 cells assessed as inhibition of conversion of [3H]-L-arginine to [3H]-L-citrulline after 45 min...More data for this Ligand-Target Pair
TargetNitric oxide synthase, endothelial(Homo sapiens (Human))
AstraZeneca R&D Charnwood
Curated by ChEMBL
AstraZeneca R&D Charnwood
Curated by ChEMBL
Affinity DataIC50: 680nMAssay Description:Inhibition of human recombinant eNOS expressed in Sf9 cells assessed as inhibition of conversion of [3H]-L-arginine to [3H]-L-citrulline after 45 min...More data for this Ligand-Target Pair
TargetNitric oxide synthase, inducible(Homo sapiens (Human))
AstraZeneca R&D Charnwood
Curated by ChEMBL
AstraZeneca R&D Charnwood
Curated by ChEMBL
Affinity DataIC50: 830nMAssay Description:Inhibition of human recombinant iNOS expressed in Sf9 cells assessed as inhibition of conversion of [3H]-L-arginine to [3H]-L-citrulline after 45 min...More data for this Ligand-Target Pair
TargetNitric oxide synthase, inducible(Mus musculus (mouse))
National Cheng Kung University
Curated by ChEMBL
National Cheng Kung University
Curated by ChEMBL
Affinity DataIC50: 2.58E+4nMAssay Description:Inhibition of iNOS in mouse BV2 microglial cells assessed as NO productionMore data for this Ligand-Target Pair
TargetNitric oxide synthase, brain(Rattus norvegicus (rat))
AstraZeneca R&D Charnwood
Curated by ChEMBL
AstraZeneca R&D Charnwood
Curated by ChEMBL
Affinity DataIC50: 150nMAssay Description:Inhibition of conversion of [3H]-L-Arg to [3H]-L-citrulline catalyzed by neuronal NOS (n NOS) from rat cerebellumMore data for this Ligand-Target Pair