null
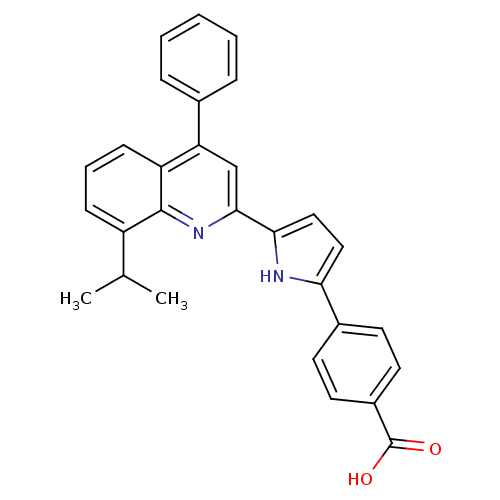
SMILES CC(C)c1cccc2c(cc(nc12)-c1ccc([nH]1)-c1ccc(cc1)C(O)=O)-c1ccccc1
InChI Key InChIKey=LSGNKLDHMQVTEK-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50099474
Found 3 hits for monomerid = 50099474
Affinity DataIC50: 31nMAssay Description:Antagonistic activity of the compound was evaluated in terms of inhibition of Retinoic acid receptor alpha transactivation by ATRA (50 nM)More data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+3nMAssay Description:Antagonistic activity of the compound was evaluated in terms of inhibition of Retinoic acid receptor beta transactivation by ATRA (50 nM); Not detect...More data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+3nMAssay Description:Antagonistic activity of the compound was evaluated in terms of inhibition of Retinoic acid receptor gamma transactivation by ATRA (50 nM); Not detec...More data for this Ligand-Target Pair