null
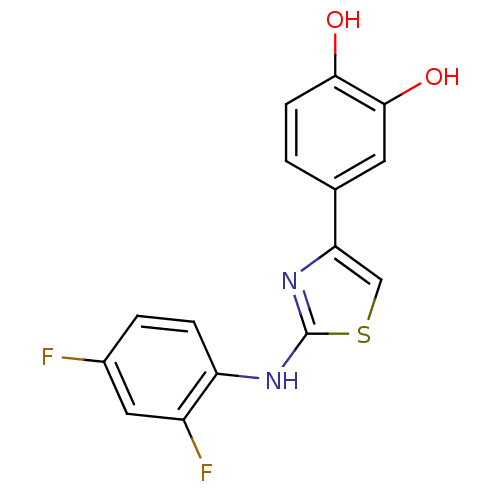
SMILES Oc1ccc(cc1O)-c1csc(Nc2ccc(F)cc2F)n1
InChI Key InChIKey=GFUKFZXSGYDXIF-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 10 hits for monomerid = 50111591
Found 10 hits for monomerid = 50111591
Affinity DataIC50: 3.20E+5nMAssay Description:Inhibition of Beta-galactosidaseMore data for this Ligand-Target Pair
Affinity DataIC50: 1.80E+4nMAssay Description:Inhibitory activity against beta-lactamaseMore data for this Ligand-Target Pair
Affinity DataIC50: 1.50E+5nMAssay Description:Inhibitory activity against cloned Dihydrofolate reductase (cDHFR)More data for this Ligand-Target Pair
Affinity DataIC50: 1.33E+5nMAssay Description:Inhibitory activity against beta-lactamase in the presence of 500 mMKPi concentration of bufferMore data for this Ligand-Target Pair
Affinity DataIC50: 1.80E+4nMAssay Description:Inhibitory activity against Amp C beta-LactamaseMore data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+3nMAssay Description:Inhibitory activity against beta-lactamase in the presence of 5 mM KPi concentration of bufferMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibitory activity against chymotrypsinogenMore data for this Ligand-Target Pair
Affinity DataIC50: 2.50E+4nMAssay Description:Inhibitory activity against malate dehydrogenase (MDH) from Thermus flavusMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibitory activity against Chymotrypsinogen from Thermus flavusMore data for this Ligand-Target Pair
Affinity DataIC50: 1.80E+4nMAssay Description:Inhibitory activity against beta-lactamase in the presence of 50 mM KPi concentration of bufferMore data for this Ligand-Target Pair