null
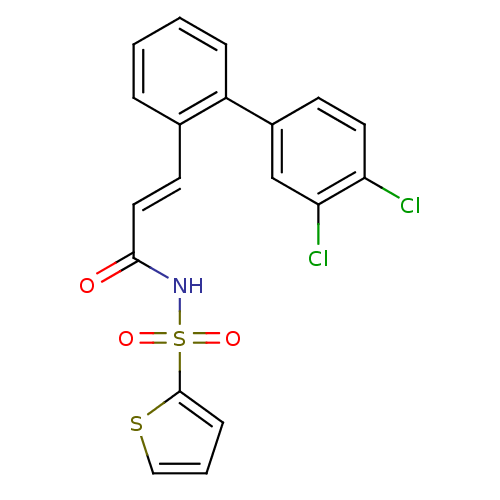
SMILES Clc1ccc(cc1Cl)-c1ccccc1\C=C\C(=O)NS(=O)(=O)c1cccs1
InChI Key InChIKey=RODREHKJZDRDIM-CSKARUKUSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 12 hits for monomerid = 50117689
Found 12 hits for monomerid = 50117689
TargetProstaglandin E2 receptor EP3 subtype(Homo sapiens (Human))
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
Affinity DataKi: 25nMAssay Description:Affinity at human Prostanoid EP3 receptor in the human embryonic kidney (HEK) 293 cell line.More data for this Ligand-Target Pair
TargetProstaglandin E2 receptor EP3 subtype(Homo sapiens (Human))
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
Affinity DataKi: 25nMAssay Description:Binding affinity at human Prostanoid EP3 receptor.More data for this Ligand-Target Pair
TargetThymidine phosphorylase(Homo sapiens (Human))
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
Affinity DataKi: 770nMAssay Description:Inhibitory constant of the compound was determined against Prostanoid TP receptorMore data for this Ligand-Target Pair
TargetProstaglandin D2 receptor(Homo sapiens (Human))
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
Affinity DataKi: 830nMAssay Description:Inhibitory constant of the compound was determined against Prostanoid DP receptorMore data for this Ligand-Target Pair
TargetProstaglandin E2 receptor EP4 subtype(Homo sapiens (Human))
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
Affinity DataKi: 3.70E+3nMAssay Description:Affinity at human Prostanoid EP4 receptor in the human embryonic kidney (HEK) 293 cell line.More data for this Ligand-Target Pair
TargetProstaglandin E2 receptor EP4 subtype(Homo sapiens (Human))
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
Affinity DataKi: 3.70E+3nMAssay Description:Binding affinity at human Prostanoid EP4 receptor.More data for this Ligand-Target Pair
TargetProstacyclin receptor(Homo sapiens (Human))
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
Affinity DataKi: 7.30E+3nMAssay Description:Inhibitory constant of the compound was determined against Prostanoid IP receptorMore data for this Ligand-Target Pair
TargetProstaglandin E2 receptor EP1 subtype(Homo sapiens (Human))
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
Affinity DataKi: 8.10E+3nMAssay Description:Affinity at human Prostanoid EP1 receptor in the human embryonic kidney (HEK) 293 cell line.More data for this Ligand-Target Pair
TargetProstaglandin E2 receptor EP1 subtype(Homo sapiens (Human))
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
Affinity DataKi: 8.20E+3nMAssay Description:Binding affinity at human Prostanoid EP1 receptor.More data for this Ligand-Target Pair
TargetProstaglandin E2 receptor EP2 subtype(Homo sapiens (Human))
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
Affinity DataKi: 1.00E+4nMAssay Description:Affinity at human Prostanoid EP2 receptor in the human embryonic kidney (HEK) 293 cell line.More data for this Ligand-Target Pair
TargetProstaglandin F2-alpha receptor(Homo sapiens (Human))
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
Affinity DataKi: 1.70E+4nMAssay Description:Inhibitory constant of the compound was determined against Prostanoid FP receptorMore data for this Ligand-Target Pair
TargetProstaglandin E2 receptor EP2 subtype(Homo sapiens (Human))
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
Affinity DataKi: 2.70E+4nMAssay Description:Binding affinity at human Prostanoid EP2 receptor.More data for this Ligand-Target Pair