null
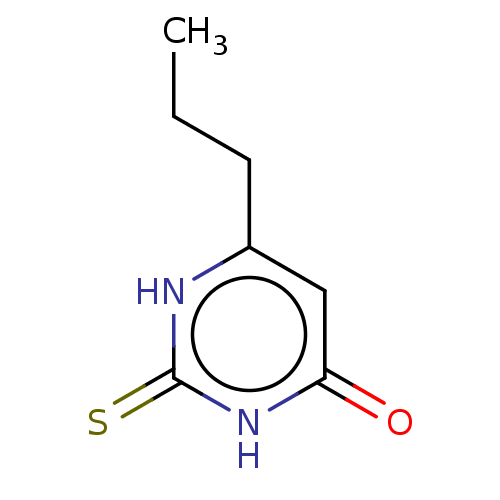
SMILES CCCc1cc(=O)[nH]c(=S)[nH]1
InChI Key InChIKey=KNAHARQHSZJURB-UHFFFAOYSA-N
PDB links: 1 PDB ID matches this monomer.
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 8 hits for monomerid = 50133597
Found 8 hits for monomerid = 50133597
Affinity DataIC50: 5.70E+3nMAssay Description:Irreversible inhibition of MPO in LPS-stimulated human whole blood after 4 hrs by Amplex Red/H2O2-based fluorescence plate reader analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 2.81E+3nMAssay Description:Inhibition of peroxidase activity of MPO isolated from human polynuclear leukocytes using Amplex Red as substrate assessed as formation of resorufin ...More data for this Ligand-Target Pair
Affinity DataIC50: 3.38E+3nMAssay Description:Inhibition of TPO (unknown origin) using Amplex Red as substrate assessed as formation of resorufin measured every 20 secs by spectrophotometric anal...More data for this Ligand-Target Pair
In DepthDetails
Affinity DataIC50: 1.33E+5nMAssay Description:Inhibition of human MRP4 overexpressed in Sf9 cell membrane vesicles assessed as uptake of [3H]-estradiol-17beta-D-glucuronide in presence of ATP and...More data for this Ligand-Target Pair
Affinity DataIC50: 1.33E+5nMAssay Description:Inhibition of human MRP2 overexpressed in Sf9 cell membrane vesicles assessed as uptake of [3H]-estradiol-17beta-D-glucuronide in presence of ATP and...More data for this Ligand-Target Pair
Affinity DataIC50: 1.33E+5nMAssay Description:Inhibition of human BSEP overexpressed in Sf9 cell membrane vesicles assessed as uptake of [3H]-taurocholate in presence of ATP measured after 15 to ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.33E+5nMAssay Description:Inhibition of human MRP3 overexpressed in Sf9 insect cell membrane vesicles assessed as uptake of [3H]-estradiol-17beta-D-glucuronide in presence of ...More data for this Ligand-Target Pair