null
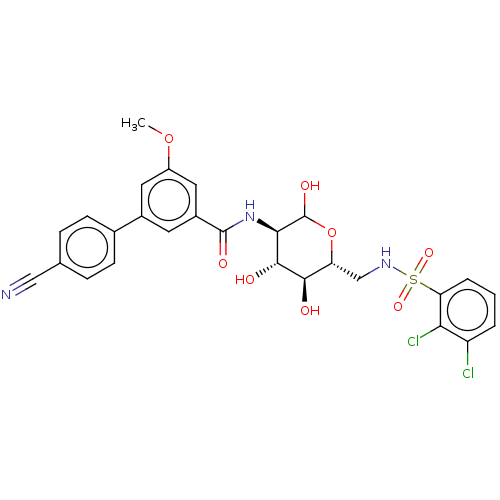
SMILES COc1cc(cc(c1)-c1ccc(cc1)C#N)C(=O)N[C@H]1C(O)O[C@H](CNS(=O)(=O)c2cccc(Cl)c2Cl)[C@@H](O)[C@@H]1O
InChI Key InChIKey=HBGMCCARHCLDCH-AREUJWMLSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50169038
Found 2 hits for monomerid = 50169038
Affinity DataIC50: 25nMAssay Description:Inhibition of His-tagged human HK2 (17 to 916 residues) expressed in Escherichia coli BL21(DE3) using glucose as substrate after 45 mins by ADP-glo a...More data for this Ligand-Target Pair
Affinity DataIC50: 7.90nMAssay Description:Inhibition of His-tagged human HK1 (11 to 917 residues) expressed in Escherichia coli BL21(DE3) using glucose as substrate after 45 mins by ADP-glo a...More data for this Ligand-Target Pair