null
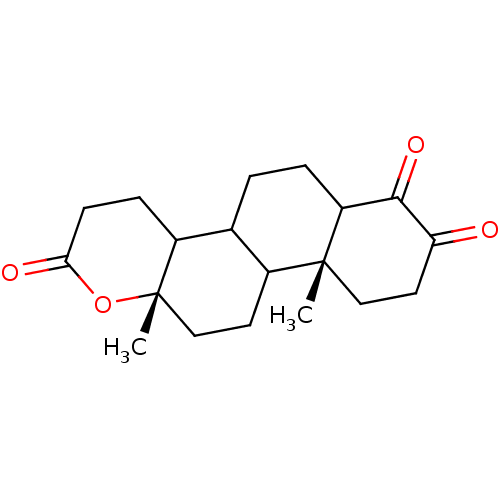
SMILES C[C@]12CCC3C(CCC4C(=O)C(=O)CC[C@]34C)C1CCC(=O)O2
InChI Key InChIKey=OQHKRXIIDQKKDS-PQBTTZHESA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50174542
Found 2 hits for monomerid = 50174542
Affinity DataKi: 660nMAssay Description:Inhibition constant against aromatase protein from human placental microsomes using [1-beta-3H]-androstenedione; Competitive inhibitionMore data for this Ligand-Target Pair
Affinity DataIC50: 1.85E+3nMAssay Description:Inhibitory concentration against aromatase protein from human placental microsomes using [1-beta-3H]-androstenedione; Competitive inhibitionMore data for this Ligand-Target Pair