null
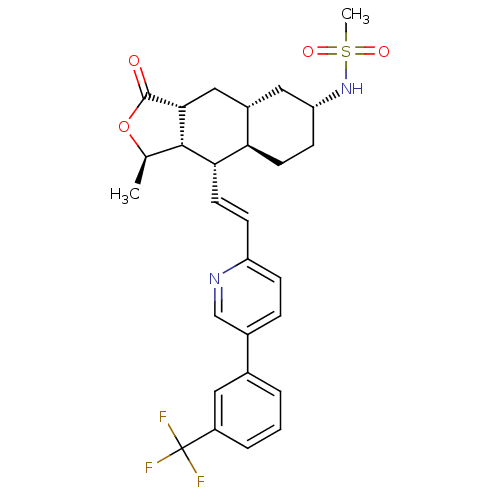
SMILES C[C@H]1OC(=O)[C@@H]2C[C@@H]3C[C@@H](CC[C@H]3[C@H](\C=C\c3ccc(cn3)-c3cccc(c3)C(F)(F)F)[C@H]12)NS(C)(=O)=O
InChI Key InChIKey=ZMJWVTFTEBJBQL-LIWLXMMZSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 1 hit for monomerid = 50261108
Found 1 hit for monomerid = 50261108
TargetProteinase-activated receptor 1(Homo sapiens (Human))
Schering-Plough Research Institute
Curated by ChEMBL
Schering-Plough Research Institute
Curated by ChEMBL
Affinity DataKi: 10nMAssay Description:Displacement of [3H]haTRAP from PAR1 in human plateletsMore data for this Ligand-Target Pair