null
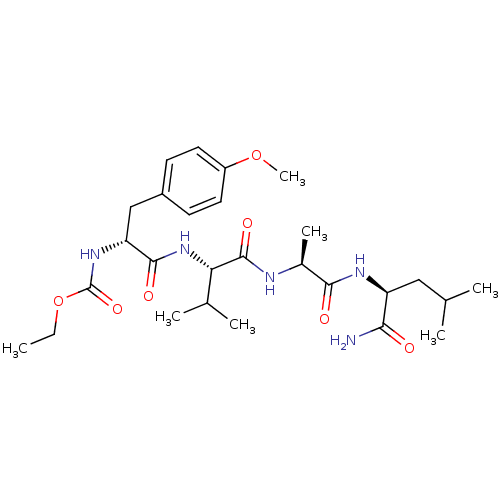
SMILES CCOC(=O)N[C@H](Cc1ccc(OC)cc1)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(C)C)C(N)=O
InChI Key InChIKey=LTPMXDWCOMDZDY-MVWVFHAYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50289280
Found 2 hits for monomerid = 50289280
Affinity DataIC50: 180nMAssay Description:Inhibitory concentration to inhibit the binding of biotinylated rat myelin basic protein peptide (RMBP90-102) against DR1 allele of class II MHC for ...More data for this Ligand-Target Pair
Affinity DataIC50: 46nMAssay Description:Inhibitory concentration to inhibit the binding of biotinylated rat myelin basic protein peptide (RMBP90-102) against DR1 allele of class II MHC for ...More data for this Ligand-Target Pair