null
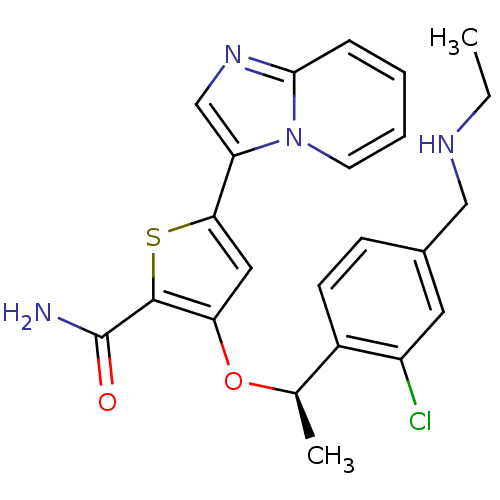
SMILES CCNCc1ccc([C@@H](C)Oc2cc(sc2C(N)=O)-c2cnc3ccccn23)c(Cl)c1
InChI Key InChIKey=ZDIQYDZTCNQUCJ-CQSZACIVSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50297969
Found 2 hits for monomerid = 50297969
TargetSerine/threonine-protein kinase PLK1(Homo sapiens (Human))
Banyu Tsukuba Research Institute
Curated by ChEMBL
Banyu Tsukuba Research Institute
Curated by ChEMBL
TargetSerine/threonine-protein kinase PLK1(Homo sapiens (Human))
Banyu Tsukuba Research Institute
Curated by ChEMBL
Banyu Tsukuba Research Institute
Curated by ChEMBL
Affinity DataEC50: 3.70E+4nMAssay Description:Inhibition of PLK1-mediated mitosis in human HeLaS3 cells after 18 hrsMore data for this Ligand-Target Pair