null
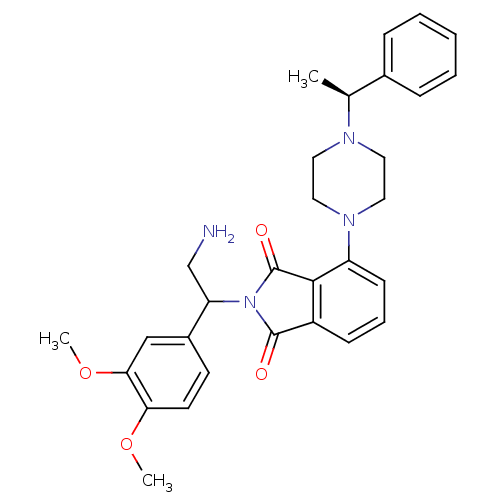
SMILES COc1ccc(cc1OC)C(CN)N1C(=O)c2cccc(N3CCN(CC3)[C@@H](C)c3ccccc3)c2C1=O
InChI Key InChIKey=GARZUTMCULGOHF-JINQPTGOSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50302234
Found 2 hits for monomerid = 50302234
TargetUrotensin-2 receptor(Homo sapiens (Human))
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
Affinity DataKi: 700nMAssay Description:Displacement of [125I]urotensin 2 from urotensin 2 receptor in human RMS13 cells by scintillation proximity assayMore data for this Ligand-Target Pair
TargetUrotensin-2 receptor(RAT)
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
Affinity DataIC50: 300nMAssay Description:Antagonist activity at rat urotensin 2 receptor expressed in CHOK1 cells assessed as inhibition of urotensin 2-induced intracellular calcium mobiliza...More data for this Ligand-Target Pair