null
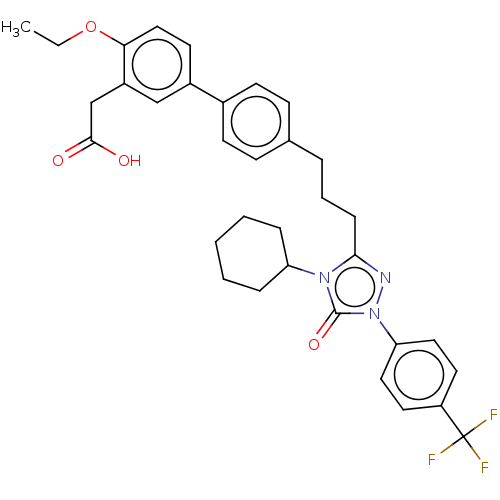
SMILES CCOc1ccc(cc1CC(O)=O)-c1ccc(CCCc2nn(-c3ccc(cc3)C(F)(F)F)c(=O)n2C2CCCCC2)cc1
InChI Key InChIKey=DCYYUFVXDNUMPU-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50508146
Found 2 hits for monomerid = 50508146
TargetPeroxisome proliferator-activated receptor delta(Homo sapiens (Human))
Inception Sciences
Curated by ChEMBL
Inception Sciences
Curated by ChEMBL
Affinity DataIC50: 66nMAssay Description:Antagonist activity at recombinant human PPARdelta assessed as inhibition of GW0742-induced receptor activation after overnight incubation by cell-ba...More data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor alpha(Homo sapiens (Human))
Inception Sciences
Curated by ChEMBL
Inception Sciences
Curated by ChEMBL
Affinity DataIC50: 66nMAssay Description:Antagonist activity at recombinant human PPARalpha assessed as inhibition of GW7647-induced receptor activation after overnight incubation by cell-ba...More data for this Ligand-Target Pair