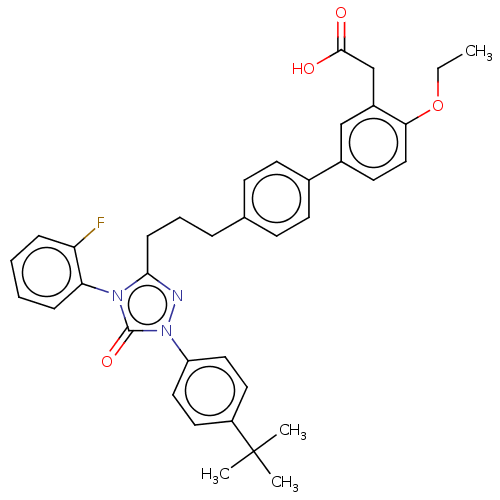
null
SMILES CCOc1ccc(cc1CC(O)=O)-c1ccc(CCCc2nn(-c3ccc(cc3)C(C)(C)C)c(=O)n2-c2ccccc2F)cc1
InChI Key InChIKey=XKCBCOVJUNVVCH-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50508151
Found 2 hits for monomerid = 50508151
TargetPeroxisome proliferator-activated receptor delta(Homo sapiens (Human))
Inception Sciences
Curated by ChEMBL
Inception Sciences
Curated by ChEMBL
Affinity DataIC50: 610nMAssay Description:Antagonist activity at recombinant human PPARdelta assessed as inhibition of GW0742-induced receptor activation after overnight incubation by cell-ba...More data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor alpha(Homo sapiens (Human))
Inception Sciences
Curated by ChEMBL
Inception Sciences
Curated by ChEMBL
Affinity DataIC50: 33nMAssay Description:Antagonist activity at recombinant human PPARalpha assessed as inhibition of GW7647-induced receptor activation after overnight incubation by cell-ba...More data for this Ligand-Target Pair